Exam 6: Ionic Bonds and Some Main-Group Chemistry
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
The third-row element having a less negative electron affinity than the elements on either side of it on the periodic table is ________.
(Short Answer)
4.7/5  (33)
(33)
Each of the pictures (a)-(d)represents one of the following substances at 25°C: potassium,fluorine,iodine,potassium fluoride,not necessarily in that order. 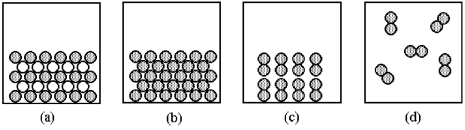 -Which picture corresponds to fluorine?
-Which picture corresponds to fluorine?
(Multiple Choice)
4.9/5  (37)
(37)
The tripositive ion with the electron configuration [Ar}3d6 is ________.
(Short Answer)
4.9/5  (31)
(31)
The following four spheres represent an Mg atom,an Mg2+ ion,a S atom,and a S2- ion,not necessarily in that order.Use your knowledge about the relative sizes of atoms,cations,and anions to determine which of the following sets of reactions is most consistent with the sizes of the atoms and ions shown below. 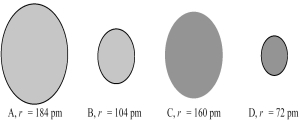
(Multiple Choice)
4.8/5  (38)
(38)
Calculate the electron affinity for the formation of the hydride ion from the following information: 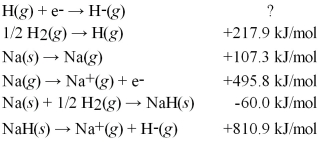
(Multiple Choice)
4.9/5  (36)
(36)
Of the following,which element has the highest first ionization energy?
(Multiple Choice)
4.8/5  (44)
(44)
The octet rule is most likely to fail occasionally for which of the following elements?
(Multiple Choice)
4.7/5  (37)
(37)
How many valence shell electrons does an atom of indium have?
(Multiple Choice)
4.9/5  (40)
(40)
![-Which element,indicated by letter on the periodic table above,has a 3+ ion with the electron configuration [Kr]4d<sup>5</sup>?](https://storage.examlex.com/TB4939/11ea7a38_c8f8_fecc_aa4c_a1e4ee487bb4_TB4939_00_TB4939_00_TB4939_00_TB4939_00.jpg) -Which element,indicated by letter on the periodic table above,has a 3+ ion with the electron configuration [Kr]4d5?
-Which element,indicated by letter on the periodic table above,has a 3+ ion with the electron configuration [Kr]4d5?
(Multiple Choice)
4.9/5  (30)
(30)
The ion Q2+ contains 10 electrons.The identity of element Q is ________.
(Short Answer)
4.9/5  (38)
(38)
What is the general trend in ionization energy and electron affinity values?
(Multiple Choice)
4.7/5  (38)
(38)
Predict the product(s)of the reaction of Br2(aq)with I-(aq).
(Multiple Choice)
4.8/5  (39)
(39)
What is the third-row element having the successive ionization energies in kJ/mol: 738,1451,7733,10,540,13,630,17,995,21,703?
(Short Answer)
4.8/5  (39)
(39)
Which of the following species will have the highest ionization energy?
(Multiple Choice)
4.9/5  (40)
(40)
Which is not generally considered to be a chemical reaction of the alkaline earth metal magnesium?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 41 - 60 of 173
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)