Exam 6: Ionic Bonds and Some Main-Group Chemistry
Exam 1: Chemistry: Matter and Measurement219 Questions
Exam 2: Atoms, molecules, and Ions257 Questions
Exam 3: Formulas, equations, and Moles208 Questions
Exam 4: Reactions in Aqueous Solutions174 Questions
Exam 5: Periodicity and the Atomic Structure of Atoms158 Questions
Exam 6: Ionic Bonds and Some Main-Group Chemistry173 Questions
Exam 7: Covalent Bonds and Molecular Structure232 Questions
Exam 8: Thermochemistry: Chemical Energy163 Questions
Exam 9: Gases: Their Properties and Behavior182 Questions
Exam 10: Liquids,solids,and Phase Changes186 Questions
Exam 11: Solutions and Their Properties192 Questions
Exam 12: Chemical Kinetics206 Questions
Exam 13: Chemical Equilibrium166 Questions
Exam 14: Aqueous Equilibria: Acids and Bases224 Questions
Exam 15: Applications of Aqueous Equilibria190 Questions
Exam 16: Thermodynamics: Entropy, free Energy, and Equilibrium144 Questions
Exam 17: Electrochemistry176 Questions
Exam 18: Hydrogen, oxygen, and Water175 Questions
Exam 19: The Main-Group Elements202 Questions
Exam 20: Transition Elements and Coordination Chemistry185 Questions
Exam 21: Metals and Solid-State Materials149 Questions
Exam 22: Nuclear Chemistry85 Questions
Exam 23: Organic and Biological Chemistry285 Questions
Select questions type
Calcium,strontium,and barium are all prepared commercially by the same method which is
(Multiple Choice)
4.9/5  (38)
(38)
What is the name for the group of elements indicated by the shaded portion of the periodic table? 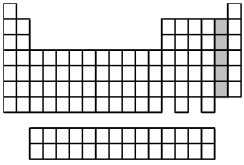
(Multiple Choice)
4.7/5  (38)
(38)
Which of the following reactions is inconsistent with the chemistry of the halogens?
(Multiple Choice)
4.7/5  (39)
(39)
An element that has the valence electron configuration 3s23p3 belongs to which period and group?
(Multiple Choice)
4.8/5  (40)
(40)
The following pictures represent alkali halide salts. 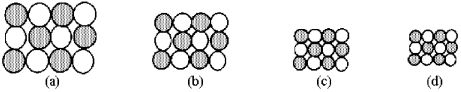 -Which salt has the lowest lattice energy?
-Which salt has the lowest lattice energy?
(Multiple Choice)
4.9/5  (33)
(33)
Which species does not have an octet of electrons for its outer core?
(Multiple Choice)
4.9/5  (27)
(27)
Which is not generally considered to be a chemical reaction of the alkaline earth metal calcium?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following ionic compounds would be expected to have the highest lattice energy?
(Multiple Choice)
4.9/5  (38)
(38)
The four spheres below represent Na+,Mg2+,F⁻,and O2-,not necessarily in that order. 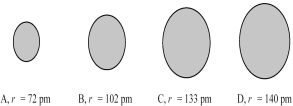 -Which sphere most likely represents the F- ion?
-Which sphere most likely represents the F- ion?
(Multiple Choice)
5.0/5  (34)
(34)
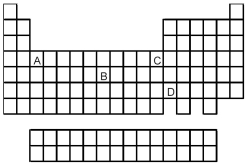 -Which element,indicated by letter on the periodic table above,has a 3+ ion with the electron configuration 1s2 2s2 2p6 3s2 3p6?
-Which element,indicated by letter on the periodic table above,has a 3+ ion with the electron configuration 1s2 2s2 2p6 3s2 3p6?
(Multiple Choice)
4.8/5  (38)
(38)
Which ionic compound would be expected to have the highest lattice energy?
(Multiple Choice)
4.8/5  (35)
(35)
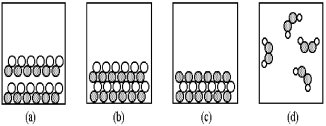 -Which of the above pictures are more likely to represent ionic compounds?
-Which of the above pictures are more likely to represent ionic compounds?
(Multiple Choice)
5.0/5  (42)
(42)
Of the following,which element has the highest first ionization energy?
(Multiple Choice)
4.7/5  (39)
(39)
Predict the product(s)when the reactants Be(s)+ Br2(l)are mixed.
(Multiple Choice)
4.9/5  (33)
(33)
Of the following,which element has the highest first ionization energy?
(Multiple Choice)
4.9/5  (38)
(38)
Which alkaline earth metal reacts the most vigorously with water at room temperature?
(Multiple Choice)
4.8/5  (39)
(39)
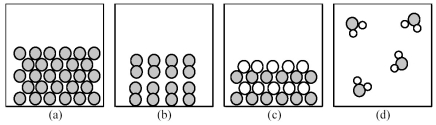 -Which of the above pictures best represents a gaseous covalent compound?
-Which of the above pictures best represents a gaseous covalent compound?
(Multiple Choice)
5.0/5  (34)
(34)
The four spheres below represent K+,Ca2+,Cl-,and S2-,not necessarily in that order. 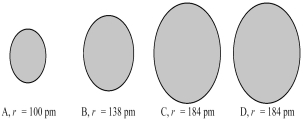 -Which sphere most likely represents the S2- ion?
-Which sphere most likely represents the S2- ion?
(Multiple Choice)
4.9/5  (42)
(42)
Showing 61 - 80 of 173
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)