Exam 2: The Matrix of Life: Weak Interactions in an Aqueous Environment
Exam 1: The Scope of Biochemistry17 Questions
Exam 2: The Matrix of Life: Weak Interactions in an Aqueous Environment25 Questions
Exam 3: The Energetics of Life25 Questions
Exam 4: Nucleic Acids28 Questions
Exam 5: Introduction to Proteins: the Primary Level of Protein Structure25 Questions
Exam 6: The Three-Dimensional Structure of Proteins24 Questions
Exam 7: Protein Function and Evolution27 Questions
Exam 8: Contractile Proteins and Molecular Motors19 Questions
Exam 9: Carbohydrates: Sugars, Saccharides, Glycans28 Questions
Exam 10: Lipids, Membranes and Cellular Transport25 Questions
Exam 11: Enzymes: Biological Catalysts24 Questions
Exam 12: Chemical Logic of Metabolism25 Questions
Exam 13: Carbohydrate Metabolism: Glycolysis, Gluconeogenesis, Glycogen Metabolism, and the Pentose Phosphate Pathway41 Questions
Exam 14: Citric Acid Cycle and Glyoxylate Cycle25 Questions
Exam 15: Electron Transport, Oxidative Phosphorylation, and Oxygen Metabolism24 Questions
Exam 16: Photosynthesis26 Questions
Exam 17: Lipid Metabolism I: Fatty Acids, Triacylglycerols, and Lipoproteins26 Questions
Exam 18: Interorgan and Intracellular Coordination of Energy Metabolism in Vertebrates22 Questions
Exam 19: Lipid Metabolism Ii: Membrane Lipids, Steroids, Isoprenoids, and Eicosanoids25 Questions
Exam 20: Metabolism of Nitrogenous Compounds I: Principles of Biosynthesis, Utilization, and Turnover25 Questions
Exam 21: Metabolism of Nitogenous Compounds II: Amino Acids, Porphyrins, and Neurotransmitters25 Questions
Exam 22: Nucleotide Metabolism25 Questions
Exam 23: Mechanisms of Signal Transduction24 Questions
Exam 24: Genes, Genomes and Chromosomes25 Questions
Exam 25: DNA Replication25 Questions
Exam 26: DNA Restructuring: Repair, Recombination, Rearrangement, Amplification25 Questions
Exam 27: Information Readout: Transcription and Post-Transcriptional Processing25 Questions
Exam 28: Information Decoding: Translation and Post-Translational Protein Processing28 Questions
Exam 29: Regulation of Gene Expression25 Questions
Select questions type
You have been asked to determine the pKa of an unknown acid. In a solution at pH 7.0, you find that 24% of the acid is in its deprotonated form. What is the pKa of the acid?
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
B
Which of the following is the conjugate acid of hydrogen phosphate, HPO42-?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
B
Glutamic acid contains two carboxylic acid groups (pKa values of 2.2 and 4.2)and an amine group (pKa 9.7). What is the pI for glutamic acid?
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
A
Use the equilibrium equation of the blood buffer to predict what would happen to blood pH if respiration were to slow significantly.
(Essay)
4.8/5  (36)
(36)
What pH range is generally considered to be the physiological pH range?
(Multiple Choice)
4.8/5  (36)
(36)
What solution conditions are required for a protein to be a positively charged macroion?
(Multiple Choice)
4.7/5  (32)
(32)
Given the structure of a glucose molecule, which of the following explains the hydrogen bonding between glucose and water? 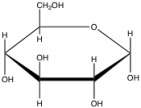
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following acids or bases is least likely to be encountered in a biochemical setting?
(Multiple Choice)
4.7/5  (40)
(40)
If a buffer is made with the pH below the pKa of the weak acid, the ratio of [base]/[acid] will be:
(Multiple Choice)
4.9/5  (23)
(23)
Which of the following atoms could interact through a hydrogen bond?
(Multiple Choice)
4.9/5  (29)
(29)
If gastric juice has a pH of about 1.5, which of the following would be predominantly deprotonated in the stomach?
(Multiple Choice)
4.9/5  (35)
(35)
Lysine contains two amine groups (pKa values of 9.0 and 10.0)and a carboxylic acid group (pKa 2.2). In a solution of pH 9.5, which of the following would best describe the protonation and charge state of lysine?
(Multiple Choice)
4.8/5  (35)
(35)
Lactic acid is a common product of actively working muscle. It is transported via the bloodstream to the liver. What percent of lactic acid is ionized in the bloodstream if the pH is 7.40 and the pKa is 3.86?
(Essay)
4.7/5  (36)
(36)
Citric acid is a triprotic acid with three carboxylic acid groups having pKa values of 3.1, 4.8, and 6.4. If a solution of citric acid has a pH of 5.5, what can be said about the predominant protonation state of the citric acid?
(Multiple Choice)
4.8/5  (33)
(33)
Since pKa = -log Ka, which of the following is a correct statement?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following best explains the hydrogen bonding that occurs in water?
(Multiple Choice)
4.9/5  (35)
(35)
Imidazole is a commonly used buffer in biochemistry labs. With a pKa of 7.0, what would be the ratio of base to acid at pH 7.4?
(Essay)
4.9/5  (36)
(36)
Which of the following would likely form micelles in an aqueous solution?
(Multiple Choice)
4.9/5  (43)
(43)
Formic acid is the active agent in an ant bite. What is the ratio of base/acid for formic acid (pKa 3.9)in the blood stream at pH 7.4?
(Multiple Choice)
4.9/5  (46)
(46)
In the following titration curve, what does the inflection point represent? 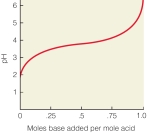
(Multiple Choice)
4.7/5  (32)
(32)
Showing 1 - 20 of 25
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)