Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Which one of the following statements about balanced equations is false? In a balanced reaction
(Multiple Choice)
5.0/5  (39)
(39)
Tablets of ascorbic acid,or Vitamin C,C6H8O6,are taken as a dietary supplement.If a typical tablet contains 500 mg,how many atoms of carbon (C)are in a tablet?
(Short Answer)
4.9/5  (36)
(36)
What is the mass of 0.0500 mol of dichlorodifluoromethane,CF2Cl2?
(Multiple Choice)
4.8/5  (36)
(36)
What mass of carbon monoxide,CO,contains the same number of molecules as 3.00 g of trichlorofluoromethane,CCl3F?
(Multiple Choice)
4.8/5  (40)
(40)
What is the empirical formula of a substance that contains 2.64 g of C,0.444 g of H,and 3.52 g of O?
(Multiple Choice)
4.8/5  (27)
(27)
How many grams of calcium chloride are needed to produce 5.00 g of potassium chloride? CaCl2(aq)+ K2CO3(aq)→ 2 KCl(aq)+ CaCO3(aq)
(Multiple Choice)
4.9/5  (30)
(30)
What is the molar mass of aspirin if 5.19 × 1016 molecules of aspirin weigh 15.53 μg?
(Multiple Choice)
4.8/5  (34)
(34)
When silver nitrate reacts with barium chloride,silver chloride and barium nitrate are formed.How many grams of silver chloride are formed when 10.8 g of silver nitrate reacts with 15.0 g of barium chloride?
(Multiple Choice)
4.7/5  (42)
(42)
When silver nitrate reacts with barium chloride,silver chloride and barium nitrate are formed.How many grams of silver chloride are formed when 8.0 g of silver nitrate reacts with 15.0 g of barium chloride?
(Multiple Choice)
4.7/5  (39)
(39)
Which statement about elemental analysis by combustion is not correct?
(Multiple Choice)
4.7/5  (33)
(33)
What is the number of moles of 5.00 × 1022 molecules of NaBr?
(Multiple Choice)
4.9/5  (41)
(41)
What is the empirical formula for ethyl fluoride if the compound contains 49.97% carbon,10.51% hydrogen,and 39.52% fluorine by mass?
(Multiple Choice)
4.8/5  (39)
(39)
If the percent yield for the following reaction is 75.0%,and 35.0 g of NO2 are consumed in the reaction,how many grams of nitric acid,HNO3(aq)are produced? 3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
(Multiple Choice)
4.9/5  (31)
(31)
The following diagram represents the reaction of A2 (unshaded spheres)with B2 (shaded spheres).How many moles of product can be produced from the reaction of 1.0 mol of A2 and 1.0 mol of B2? 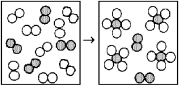
(Multiple Choice)
4.8/5  (39)
(39)
![-Diethyl ether has the molecular formula C<sub>4</sub>H<sub>10</sub>O.Which ball and stick model shown above represents diethyl ether? [gray spheres = C,black spheres = O,unshaded spheres = H]](https://storage.examlex.com/TB4940/11ea7e2d_d09e_aff7_a2f7_11edcd81f00c_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg) -Diethyl ether has the molecular formula C4H10O.Which ball and stick model shown above represents diethyl ether? [gray spheres = C,black spheres = O,unshaded spheres = H]
-Diethyl ether has the molecular formula C4H10O.Which ball and stick model shown above represents diethyl ether? [gray spheres = C,black spheres = O,unshaded spheres = H]
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following statements is false concerning the formula of a compound?
(Multiple Choice)
4.9/5  (31)
(31)
The density of ethanol,C2H5OH,is 0.789 g/mL.How many milliliters of ethanol are needed to produce 5.00 g of CO2 according to the following chemical equation?
C2H5OH(l)+ 3 O2(g)→ 2 CO2(g)+ 3 H2O(l)
(Multiple Choice)
4.9/5  (31)
(31)
Dinitrogen monoxide gas decomposes to form nitrogen gas and oxygen gas.How many grams of nitrogen are formed when 30.00 g of dinitrogen monoxide decomposes?
(Multiple Choice)
4.9/5  (39)
(39)
Showing 81 - 100 of 159
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)