Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
![-Ethanol has the molecular formula C<sub>2</sub>H<sub>6</sub>O.Which ball and stick model shown above represents ethanol? [gray spheres = C,black spheres = O,unshaded spheres = H]](https://storage.examlex.com/TB4940/11ea7e2d_d09e_aff7_a2f7_11edcd81f00c_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg) -Ethanol has the molecular formula C2H6O.Which ball and stick model shown above represents ethanol? [gray spheres = C,black spheres = O,unshaded spheres = H]
-Ethanol has the molecular formula C2H6O.Which ball and stick model shown above represents ethanol? [gray spheres = C,black spheres = O,unshaded spheres = H]
(Multiple Choice)
4.8/5  (36)
(36)
Strontium phosphate reacts with sulfuric acid to form strontium sulfate and phosphoric acid.What is the coefficient for sulfuric acid when the equation is balanced using the lowest whole-numbered coefficients?
(Multiple Choice)
4.9/5  (37)
(37)
Oxygen can be produced from the catalytic decomposition of KClO3 as shown in the balanced equation below.
2 KClO3 → 2 KCl + 3 O2
What is the percent yield if 3.20 grams of oxygen are formed from the reaction of 12.3 grams of KClO3?
(Short Answer)
4.8/5  (33)
(33)
How many grams of calcium chloride are needed to produce 10.0 g of potassium chloride? CaCl2(aq)+ K2CO3(aq)→ 2 KCl(aq)+ CaCO3(aq)
(Multiple Choice)
4.9/5  (25)
(25)
The balanced equation for the decomposition of water is shown below.
2 H2O → 2 H2 + O2
If 0.72 g of water react completely in this reaction,what is the theoretical yield of H2?
(Short Answer)
4.8/5  (37)
(37)
What is the balanced chemical equation for the reaction of element A (unshaded spheres)with element B (shaded spheres)as represented below? 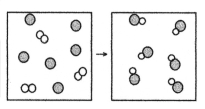
(Multiple Choice)
4.9/5  (39)
(39)
The following diagram represents the reaction of A (unshaded spheres)with B (shaded spheres).What is the balanced chemical equation for this reaction,and what is the limiting reactant? 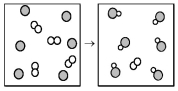
(Multiple Choice)
4.9/5  (38)
(38)
5.0 g of nitrogen is reacted with 5.0 g of hydrogen to produce ammonia according to the chemical equation shown below.Which one of the following statements is false?
N2(g)+ 3 H2(g)→ 2 NH3(g)
(Multiple Choice)
4.7/5  (26)
(26)
How many oxygen atoms are there in 6.00 g of sodium dichromate,Na2Cr2O7?
(Multiple Choice)
4.9/5  (45)
(45)
When carbon dioxide dissolves in water,H+ is formed,which makes the solution acidic,as shown in the balanced equation below.
CO2 + H2O → HCO3- + H+
What is the percent yield if 0.0088 g of CO2 reacts with 900 g of H2O to form 0.000108 g of H+?
(Short Answer)
4.7/5  (44)
(44)
Isoeugenol is the compound which gives the characteristic odor to nutmeg and contains carbon,hydrogen,and oxygen.If a 0.500 g sample of isoeugenol is combusted it gives 1.341 g of CO2 and 0.329 g of H2O.Isoeugenol has a molecular weight of 164 g/mol.What is the molecular formula of isoeugenol?
(Multiple Choice)
4.9/5  (36)
(36)
Balance the chemical equation given below,and determine the number of grams of MgO are needed to produce 25.0 g of Fe2O3. ________ MgO(s)+ ________ Fe(s)→ ________ Fe2O3(s)+ ________ Mg(s)
(Multiple Choice)
4.9/5  (31)
(31)
Balance the chemical equation given below,and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 9.00 g of oxygen at 25°C? The density of nitrogen monoxide at 25°C is 1.23 g/L. ________ NH3(g)+ ________ O2(g)→ ________ NO(g)+ ________ H2O(l)
(Multiple Choice)
4.7/5  (45)
(45)
Balance the chemical equation given below,and determine the number of moles of iodine that reacts with 10.0 g of aluminum. ________ Al(s)+ ________ I2(s)→ ________ Al2I6(s)
(Multiple Choice)
4.7/5  (35)
(35)
Showing 121 - 140 of 159
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)