Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
![-Acetone has the formula C<sub>3</sub>H<sub>6</sub>O.Which ball and stick model shown above represents acetone? [gray spheres = C,black spheres = O,unshaded spheres = H]](https://storage.examlex.com/TB4940/11ea7e2d_d09e_aff7_a2f7_11edcd81f00c_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg) -Acetone has the formula C3H6O.Which ball and stick model shown above represents acetone? [gray spheres = C,black spheres = O,unshaded spheres = H]
-Acetone has the formula C3H6O.Which ball and stick model shown above represents acetone? [gray spheres = C,black spheres = O,unshaded spheres = H]
(Multiple Choice)
4.8/5  (40)
(40)
Combustion analysis of a 0.675 g sample of an unknown compound that contains only carbon,hydrogen,and oxygen gives 0.627 g of CO2 and 1.534 g of H2O.The molecular mass of the unknown is
(Multiple Choice)
4.8/5  (39)
(39)
How many chloride ions are there in 3.00 mol of aluminum chloride?
(Multiple Choice)
4.8/5  (30)
(30)
The following diagrams represent the reaction of A2 (shaded spheres)with B2 (unshaded spheres).How many moles of product can be made from 1.0 mol of A2 and 1.0 mol of B2? 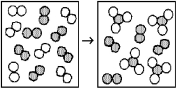
(Multiple Choice)
4.9/5  (34)
(34)
When the reaction C4H10 + O2 → CO2 + H2O is balanced using the smallest whole number coefficients,the coefficient in front of O2 is ________.
(Short Answer)
4.8/5  (34)
(34)
The empirical formula of a compound that contains 82.66% carbon and 17.34% hydrogen is ________.
(Short Answer)
4.9/5  (33)
(33)
Analysis of a 1.000-g sample of the oral hypoglycemic agent metformintm yielded 0.3720 g of carbon,0.0858 g of hydrogen,and 0.5422 g of nitrogen.Metformintm has a molar mass of 129.16 g/mol.What is the molecular formula of Metformintm?
(Short Answer)
4.8/5  (42)
(42)
What is the stoichiometric coefficient for oxygen when the following equation is balanced using the lowest whole-number coefficients? ________ C3H6O2(l)+ ________ O2(g)→ ________ CO2(g)+ ________ H2O(l)
(Multiple Choice)
4.9/5  (29)
(29)
Dinitrogen monoxide gas decomposes to form nitrogen gas and oxygen gas.How many grams of oxygen are formed when 20.0 g of dinitrogen monoxide decomposes?
(Multiple Choice)
4.8/5  (36)
(36)
If the percent yield for the following reaction is 60.0%,and 45.0 g of NO2 are consumed in the reaction,how many grams of nitric acid,HNO3(aq),are produced?
3 NO2(g)+ H2O(l)→ 2 HNO3(aq)+ NO(g)
(Multiple Choice)
4.9/5  (47)
(47)
If unshaded spheres represent nitrogen atoms and shaded spheres represent oxygen atoms,which box represents reactants and which represents products for the reaction 2 NO2(g)→ 2 NO(g)+ O2(g)? 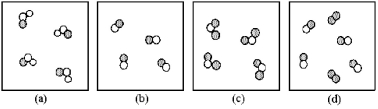
(Multiple Choice)
4.8/5  (33)
(33)
The number of grams in 0.250mol of urea, (NH2)2CO,is ________.
(Short Answer)
4.9/5  (34)
(34)
What is the stoichiometric coefficient for water when the following equation is balanced using the lowest whole-number coefficients? ________ C3H8O(l)+ ________ O2(g)→ ________ CO2(g)+ ________ H2O(l)
(Multiple Choice)
4.8/5  (32)
(32)
If unshaded spheres represent nitrogen atoms and shaded spheres represent oxygen atoms,which box represents reactants and which represents products for the reaction 2 N2O(g)→ 2 N2(g)+ O2(g)? 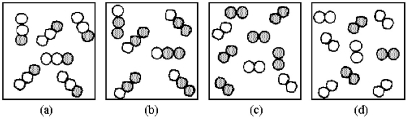
(Multiple Choice)
5.0/5  (42)
(42)
Reaction of A (unshaded spheres)with B2 (shaded spheres)is shown schematically in the following diagram.Which equation best describes the stoichiometry of the reaction? 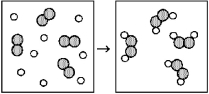
(Multiple Choice)
4.7/5  (33)
(33)
Showing 101 - 120 of 159
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)