Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
If the percent yield for the following reaction is 65.0%,how many grams of KClO3 are needed to produce 42.0 g of O2? 2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
(Multiple Choice)
4.7/5  (45)
(45)
How many moles of CuO can be produced from 1.80 mol of Cu2O in the following reaction? 2 Cu2O(s)+ O2(g)→ 4 CuO(s)
(Multiple Choice)
4.9/5  (37)
(37)
What is the balanced chemical equation for the reaction of element A (unshaded spheres)with element B (shaded spheres)as represented below? 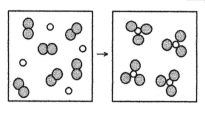
(Multiple Choice)
5.0/5  (40)
(40)
Aluminum metal reacts with aqueous copper(II)sulfate to form aqueous aluminum sulfate and copper metal.What is the stoichiometric coefficient for aluminum when the chemical equation is balanced using the lowest whole-number stoichiometric coefficients?
(Multiple Choice)
4.8/5  (34)
(34)
To the nearest whole number,the molar mass of Cu(NO3)2 is ________ g/mol.
(Short Answer)
4.9/5  (41)
(41)
What is the balanced chemical equation for the reaction of element A (unshaded spheres)with element B (shaded spheres)as represented below? 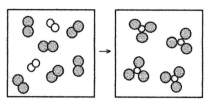
(Multiple Choice)
4.8/5  (41)
(41)
Hydrazine,N2H4,once used as a rocket propellant,reacts with oxygen in the following equation. N2H4(g)+ O2(g)→ N2(g)+ 2 H2O(g)
The reaction,at 50% yield,produces 4.0 moles of N2.What was the mass of hydrazine used? Assume that O2 is in excess.
(Multiple Choice)
4.8/5  (25)
(25)
Tablets of ascorbic acid,or Vitamin C,C6H8O6,are taken as a dietary supplement.If a typical tablet contains 500 mg,how many molecules of Vitamin C are in a tablet?
(Multiple Choice)
4.9/5  (32)
(32)
What is the balanced chemical equation for the reaction of element A (unshaded spheres)with element B (shaded spheres)as represented below? 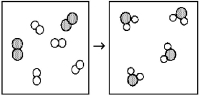
(Multiple Choice)
4.8/5  (31)
(31)
What mass of sulfur hexafluoride,SF6,has the same number of fluorine atoms as 50.0 g of oxygen difluoride,OF2?
(Multiple Choice)
4.8/5  (33)
(33)
What is the empirical formula for perfluoropropane if the compound contains 81% fluorine and 19% carbon by mass?
(Multiple Choice)
4.9/5  (39)
(39)
Which substance is the limiting reactant when 16.0 g of sulfur reacts with 10.0 g of oxygen and 12.0 g of sodium hydroxide according to the following chemical equation? 2 S(s)+ 3 O2(g)+ 4 NaOH(aq)→ 2 Na2SO4(aq)+ 2 H2O(l)
(Multiple Choice)
4.9/5  (33)
(33)
What mass of sulfur hexafluoride,SF6,has the same number of fluorine atoms as 25.0 g of oxygen difluoride,OF2?
(Multiple Choice)
4.7/5  (31)
(31)
When iron(III)oxide reacts with hydrochloric acid,iron(III)chloride and water are formed.How many grams of iron(III)chloride are formed from 10.0 g of iron(III)oxide and 10.0 g of hydrochloric acid?
(Multiple Choice)
4.9/5  (35)
(35)
What is the molar mass of pentane if 4.18 × 1016 molecules of pentane weigh 5.00 μg?
(Multiple Choice)
4.9/5  (36)
(36)
3.0 moles of nitrogen is reacted with 11.0 moles of hydrogen to produce ammonia according to the chemical equation shown below.Which one of the following statements is false? N2(g)+ 3 H2(g)→ 2 NH3(g)
(Multiple Choice)
4.8/5  (48)
(48)
The following diagrams represent the reaction of A2 (shaded spheres)with B2 (unshaded spheres).Identify the limiting reactant and write a balanced equation for the reaction. 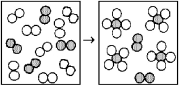
(Multiple Choice)
4.8/5  (28)
(28)
Showing 61 - 80 of 159
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)