Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
When 5.00 × 1022 molecules of ammonia react with 4.00 × 1022 molecules of oxygen according to the chemical equation shown below,how many grams of nitrogen gas are produced? 4 NH3(g)+ 3 O2(g)→ 2 N2(g)+ 6 H2O(g)
(Multiple Choice)
4.7/5  (34)
(34)
When methane,CH4,undergoes combustion with oxygen,the usual products are carbon dioxide and water.Carbon monoxide is formed when the limiting reactant is
(Multiple Choice)
4.8/5  (40)
(40)
Combustion analysis of an unknown compound containing only carbon and hydrogen produced 1.1385 g of CO2 and 0.5805 g of H2O.What is the empirical formula of the compound?
(Multiple Choice)
4.9/5  (35)
(35)
![-2-Propanol has the molecular formula C<sub>3</sub>H<sub>8</sub>O.Which ball and stick model shown above represents 2-propanol? [gray spheres = C,black spheres = O,unshaded spheres = H]](https://storage.examlex.com/TB4940/11ea7e2d_d09e_aff7_a2f7_11edcd81f00c_TB4940_00_TB4940_00_TB4940_00_TB4940_00.jpg) -2-Propanol has the molecular formula C3H8O.Which ball and stick model shown above represents 2-propanol? [gray spheres = C,black spheres = O,unshaded spheres = H]
-2-Propanol has the molecular formula C3H8O.Which ball and stick model shown above represents 2-propanol? [gray spheres = C,black spheres = O,unshaded spheres = H]
(Multiple Choice)
4.8/5  (48)
(48)
The balanced equation for the gaseous state oxidation of ammonia is shown below.
4 NH3 + 5 O2 → 4 NO + 6 H2O
How many moles of O2 are required to react with 1.2 mole of NH3?
(Short Answer)
4.8/5  (38)
(38)
How many grams of the excess reagent are left over when 6.00 g of CS2 gas react with 10.0 g of Cl2 gas in the following reaction? CS2(g)+ 3 Cl2(g)→ CCl4(l)+ S2Cl2(l)
(Multiple Choice)
4.8/5  (36)
(36)
Each molecule of cortisone contains 21 atoms of carbon (plus other atoms).The mass percentage of carbon in cortisone is 69.98%.What is the molar mass of cortisone?
(Multiple Choice)
4.8/5  (37)
(37)
A balanced equation has the same numbers and kinds of ________ on both sides of the reaction arrow.
(Short Answer)
4.8/5  (34)
(34)
The following diagram represents the reaction of A2 (unshaded spheres)with B (shaded spheres).How many moles of product can be produced from the reaction of 1.0 mol of A2 and 1.0 mol of B? 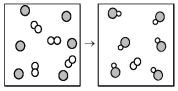
(Multiple Choice)
4.8/5  (30)
(30)
How many moles of BCl3 are needed to produce 15.0 g of HCl(aq)in the following reaction? BCl3(g)+ 3 H2O(l)→ 3 HCl(aq)+ B(OH)3(aq)
(Multiple Choice)
4.9/5  (37)
(37)
How many sodium atoms are in 3.00 g of sodium dichromate,Na2Cr2O7?
(Multiple Choice)
4.9/5  (35)
(35)
Consider two reactants,A and B.The molar mass of A is greater han the molar mass of B.You add equal masses of A and B together and let them react.Which of the following statements must be true?
(Multiple Choice)
4.8/5  (37)
(37)
1.00 mole of O2 contains the same number of oxygen atoms as
(Multiple Choice)
4.8/5  (33)
(33)
The following diagrams represent the reaction of A2 (shaded spheres)with B2 (unshaded spheres).Identify the limiting reactant and write a balanced equation for the reaction. 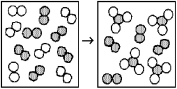
(Multiple Choice)
4.8/5  (42)
(42)
Showing 21 - 40 of 159
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)