Exam 3: Mass Relationships in Chemical Reactions
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Dinitrogen monoxide gas decomposes to form nitrogen gas and oxygen gas.How many grams of nitrogen are formed when 5.54 g of dintrogen monoxide decomposes?
(Multiple Choice)
4.8/5  (42)
(42)
The following diagram represents the reaction of A2 (unshaded spheres)with B (shaded spheres).What is the balanced chemical equation for this reaction,and what is the limiting reactant? 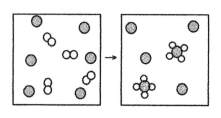
(Multiple Choice)
4.8/5  (23)
(23)
How many moles of H2O are needed to produce 15.0 g of HCl(aq)in the following reaction? BCl3(g)+ 3 H2O(l)→ 3 HCl(aq)+ B(OH)3(aq)
(Multiple Choice)
4.8/5  (48)
(48)
The balanced equation for the reaction of acetylene,C2H2,and oxygen in an acetylene torch is
2 C2H2 + 5 O2 → 4 CO2 + 2 H2O.
In this reaction the number of grams of oxygen required to react with 0.13 g of acetylene is ________.
(Short Answer)
4.8/5  (45)
(45)
In the reaction between glucose and oxygen,10.0 g of glucose reacts and 7.50 L of carbon dioxide is formed.What is the percent yield if the density of CO2 is 1.26 g/L?
C6H12O6(s)+ 6 O2(g)→ 6 CO2(g)+ 6 H2O(l)
(Multiple Choice)
4.8/5  (32)
(32)
Given the chemical equation: N2 + 3 H2 → 2 NH3.On a molecular level,what do the coefficients mean?
(Multiple Choice)
4.9/5  (45)
(45)
Balance the chemical equation given below,and determine the number of moles of iodine that reacts with 5.0 g of aluminum. ________ Al(s)+ ________ I2(s)→ ________ Al2I6(s)
(Multiple Choice)
4.7/5  (34)
(34)
Hydrazine,N2H4,is used as a rocket fuel.In the reaction below,if 80.1 g of N2H4 and 92.0 g of N2O4 are allowed to react,which is the limiting reactant,and how many grams of excess reactant remain at the end of the reaction?
2 N2H4 + N2O4 → 3 N2 + 4 H2O
(Essay)
4.8/5  (39)
(39)
4.0 g of iron is reacted with 4.0 g of water according to the chemical equation shown below.Which one of the following statements is false? 3 Fe(s)+ 4 H2O(l)→ Fe3O4(s)+ 4 H2(g)
(Multiple Choice)
4.7/5  (30)
(30)
Combustion analysis of 2.796 g of an unknown compound containing carbon,hydrogen,and oxygen produced 5.597 g of CO2 and 2.268 g of H2O.What is the empirical formula of the compound?
(Multiple Choice)
4.9/5  (40)
(40)
Combustion analysis of an unknown compound containing only carbon and hydrogen produced 2.845 g of CO2 and 1.744 g of H2O.What is the empirical formula of the compound?
(Multiple Choice)
4.8/5  (37)
(37)
What is the identity of substance X if 0.380 mol of X weighs 17.5 g?
(Multiple Choice)
4.9/5  (30)
(30)
Which one of the following compounds contains the smallest percent oxygen by mass?
(Multiple Choice)
4.8/5  (38)
(38)
Chemical equations are balanced in order to obey the law of
(Multiple Choice)
4.9/5  (34)
(34)
Which one of the following statements about balanced equations is true? A reaction is balanced by
(Multiple Choice)
4.9/5  (34)
(34)
Balance the chemical equation given below,and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 14.0 g of oxygen at 25°C? The density of nitrogen monoxide at 25°C is 1.23 g/L. ________ NH3(g)+ ________ O2(g)→ ________ NO(g)+ ________ H2O(l)
(Multiple Choice)
4.7/5  (37)
(37)
Showing 41 - 60 of 159
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)