Exam 10: Gases: Their Properties and Behavior
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Which of the following regions of the earth's atmosphere contains the ozone layer?
(Multiple Choice)
4.8/5  (34)
(34)
A basketball is inflated to a pressure of 2.10 atm in a 20.0°C garage.What is the pressure of the basketball outside where the temperature is -5.00°C?
(Multiple Choice)
4.8/5  (42)
(42)
In an open end manometer,one end of a U-tube filled with mercury is attached to a gas-filled container and the other end is open to the atmosphere.If the gas pressure in the container is less than atmospheric pressure
(Multiple Choice)
4.9/5  (44)
(44)
A 1.00 L flask contains nitrogen gas at 25°C and 1.00 atm pressure.What is the final pressure in the flask if an additional 0.50 g of N2 gas is added to the flask and the flask cooled to -55°C?
(Multiple Choice)
4.8/5  (29)
(29)
When pressure-volume measurements are made on 1.0 mol of gas at constant temperature,a plot V versus P results in a
(Multiple Choice)
4.8/5  (26)
(26)
A balloon filled with helium gas at 20°C occupies 2.91 L at 1.00 atm.The balloon is immersed in liquid nitrogen at -180°C,raising the pressure to 5.20 atm.What is the volume of the balloon in the liquid nitrogen?
(Multiple Choice)
4.8/5  (42)
(42)
If the number of moles of gas is halved at constant temperature and volume,the pressure of the gas
(Multiple Choice)
4.9/5  (36)
(36)
If 1.0 gram each of Cl2,CO2,N2,and O2 are contained in a 5-L flask,the gas with the highest partial pressure is ________.
(Short Answer)
4.7/5  (43)
(43)
Which of the following equations represents "Boyle's law"?
(Multiple Choice)
4.8/5  (35)
(35)
The apparatus shown is called a closed-end manometer because the arm not connected to the gas sample is closed to the atmosphere and is under vacuum. 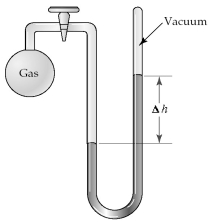 -What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 745 mm Hg and the difference in mercury levels,Δh,is 25 mm Hg?
-What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 745 mm Hg and the difference in mercury levels,Δh,is 25 mm Hg?
(Multiple Choice)
4.8/5  (50)
(50)
The mixing of different gases by random molecular motion with frequent collisions is called
(Multiple Choice)
4.8/5  (26)
(26)
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains He,cylinder B contains CO2,and cylinder C contains SO2.According to the kinetic molecular theory,which gas has the highest density?
(Multiple Choice)
4.9/5  (45)
(45)
A 0.529-g sample of gas occupies 125 mL at 60.cm of Hg and 25°C.What is the molar mass of the gas?
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following regions of the earth's atmosphere is farthest from the surface of the earth?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following is the principal cause of global warming?
(Multiple Choice)
4.9/5  (31)
(31)
A greenhouse gas of greatest concern that is from human activities is
(Multiple Choice)
4.9/5  (44)
(44)
A gas bottle contains 0.450 mol of gas at 730 mm Hg pressure.If the final pressure is 1.15 atm,how many moles of gas were added to the bottle?
(Multiple Choice)
4.7/5  (38)
(38)
Showing 121 - 140 of 188
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)