Exam 10: Gases: Their Properties and Behavior
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
An unknown gas contains 83% C and 17% H by mass.If effuses at 0.87 times the rate of CO2 gas under the same conditions.What is the molecular formula of the unknown gas?
(Multiple Choice)
4.8/5  (46)
(46)
The molecule believed to be most responsible for global warming is ________.
(Short Answer)
4.9/5  (25)
(25)
How many liters of SO3(g)are produced at 25°C and 1.00 atm from the combustion of 1.00 kg of coal which is 1.00% S by weight? Assume all the sulfur in the coal ends up as SO3.
(Multiple Choice)
5.0/5  (41)
(41)
A glass tube has one end in a dish of mercury and the other end closed by a stopcock.The distance from the surface of the mercury to the bottom of the stopcock is 800 mm,as indicated by the meter stick shown in the drawing below.The apparatus is at 20°C,and the mercury level in the tube is the same as that in the dish. 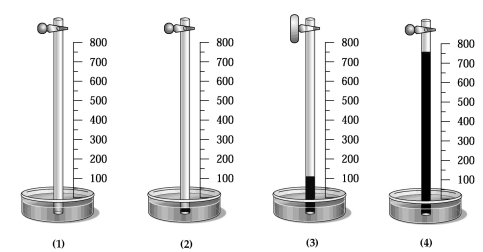 -Which drawing best shows the approximate level of the mercury in the tube when the temperature of the entire apparatus is lowered from +20°C to -20°C?
-Which drawing best shows the approximate level of the mercury in the tube when the temperature of the entire apparatus is lowered from +20°C to -20°C?
(Multiple Choice)
4.9/5  (51)
(51)
What is the Celsius temperature of 100.0 g of chlorine gas in a 45.0-L container at 800 mm Hg?
(Multiple Choice)
4.8/5  (31)
(31)
Effusion of a 1:1 mixture of two gases,represented by unshaded and shaded spheres in the diagram below,through a small pinhole produces the result shown below.The shaded spheres have a molecular mass of 32 amu.Which gas molecules have the higher average speed and what is the molecular mass of the unshaded molecules? 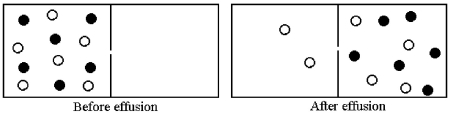
(Multiple Choice)
4.9/5  (38)
(38)
If the pressure in a gas container that is connected to an open-end U-tube manometer is 126 kPa and the pressure of the atmosphere at the open end of the tube is 732 mm Hg,the level of mercury in the tube will
(Multiple Choice)
4.8/5  (26)
(26)
Gases do not behave ideally under conditions of ________ pressure and ________ temperature.
(Short Answer)
4.9/5  (40)
(40)
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1).The initial pressure,number of moles,and temperature of the gas are noted on the diagram. 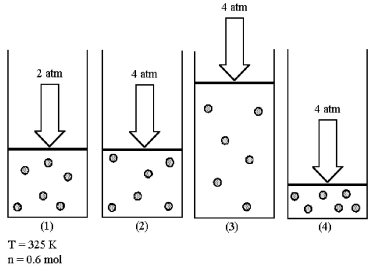 -Which diagram (2)-(4)most closely represents the result of doubling the pressure and doubling the temperature while keeping the number of moles of gas constant?
-Which diagram (2)-(4)most closely represents the result of doubling the pressure and doubling the temperature while keeping the number of moles of gas constant?
(Multiple Choice)
5.0/5  (40)
(40)
The pressure in the eye of a hurricane is less than atmospheric pressure.Which one of the following pressure readings could not have been taken in the eye of a hurricane?
(Multiple Choice)
4.8/5  (33)
(33)
You are given two flasks of equal volume.One contains H2 at 0°C and 1 atm while the other contains CO2 at 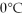 and 2 atm.Which of the following quantities will be the same for both flasks?
and 2 atm.Which of the following quantities will be the same for both flasks?
(Multiple Choice)
4.8/5  (43)
(43)
If the pressure in a gas container that is connected to an open-end U-tube manometer is 106 kPa and the pressure of the atmosphere at the open end of the tube is 700 mm Hg,the level of mercury in the tube will be
(Multiple Choice)
4.9/5  (25)
(25)
According to Graham's law,the rate of effusion of a gas is inversely proportional to the ________.
(Short Answer)
4.7/5  (36)
(36)
Some assumptions from the kinetic molecular theory are listed below.Which one is most frequently cited to explain diffusion of a gas?
(Multiple Choice)
4.8/5  (37)
(37)
In the laboratory,hydrogen gas is usually made by the following reaction: Zn(s)+ 2 HCl(aq)→ H2(g)+ ZnCl2(aq)
How many liters of H2 gas,collected over water at an atmospheric pressure of 752 mm Hg and a temperature of 21.0°C,can be made from 2.566 g of Zn and excess HCl? The partial pressure of water vapor is 18.65 mm Hg at 21.0°C.
(Multiple Choice)
4.9/5  (35)
(35)
At 298 K which is larger rate of effusion or rate of diffusion?
(Short Answer)
4.7/5  (38)
(38)
When 12.0 g of zinc metal reacts with excess HCl,how many liters of H2 gas are produced at STP?
(Multiple Choice)
4.8/5  (34)
(34)
A container filled with gas is connected to an open-end U-tube manometer that is filled with mineral oil.The pressure in the gas container is 770 mm Hg and atmospheric pressure is 754 mm Hg.What will be the difference in the levels of mineral oil in the two arms of the manometer if the densities of Hg and mineral oil are 13.6 g/mL and 0.822 g/mL respectively?
(Multiple Choice)
4.7/5  (45)
(45)
Showing 101 - 120 of 188
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)