Exam 10: Gases: Their Properties and Behavior
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
What is the total pressure in a 8.00-L flask which contains 0.127 mol of H2(g)and 0.288 mol of N2(g)at 20.0°C?
(Multiple Choice)
4.7/5  (35)
(35)
When 30.0 g of zinc metal reacts with excess HCl,how many liters of H2 gas are produced at STP?
(Multiple Choice)
4.7/5  (32)
(32)
A greenhouse gas is a gas that can experience a change in dipole moment.Which of the following can be greenhouse gases?
(Multiple Choice)
4.9/5  (43)
(43)
What is the average speed (actually the root-mean-square speed)of a carbon monoxide molecule at 27°C?
(Multiple Choice)
4.9/5  (37)
(37)
A 75.0 L steel tank at 20.0°C contains acetylene gas,C2H2,at a pressure of 1.20 atm.Assuming ideal behavior,how many grams of acetylene are in the tank?
(Multiple Choice)
4.9/5  (35)
(35)
Which one of the following gases will have the highest rate of effusion?
(Multiple Choice)
4.9/5  (33)
(33)
One mole of gas at 25°C in a 3.0-L flask has a ________ pressure than one mole of gas at 25°C in a 14.0-L flask.
(Short Answer)
4.7/5  (40)
(40)
In the diagram below,nitrogen molecules are represented by unshaded spheres,oxygen molecules by gray spheres,and chlorine molecules by black spheres.  -If the total pressure in the container is 400.mm Hg,what is the partial pressure of nitrogen?
-If the total pressure in the container is 400.mm Hg,what is the partial pressure of nitrogen?
(Multiple Choice)
4.8/5  (44)
(44)
According to the kinetic molecular theory of gases,what is the force of attraction of one CO molecule for another CO molecule?
(Essay)
4.9/5  (33)
(33)
Hydrogen gas is collected over water in an inverted buret.If the atmospheric pressure is 745 mm Hg,the vapor pressure of water is 18 mm Hg,and a 15.0 cm-high column of water remains in the buret,the pressure of the hydrogen gas is
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following substances has increased markedly due to the use of fossil fuels and contributes to the greenhouse effect?
(Multiple Choice)
4.9/5  (34)
(34)
Refrigeration and air conditioning are a source of greenhouse gases such as
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following instruments directly measures the pressure of a gas?
(Multiple Choice)
4.7/5  (43)
(43)
How many grams of CO gas are there in a 5.00-L cylinder at 4.00 × 103 mm Hg and 23°C?
(Multiple Choice)
4.9/5  (40)
(40)
Three identical flasks contain three different gases at standard temperature and pressure.Flask A contains Ar,flask B contains Ne,flask C contains H2.Which flask contains the largest number of molecules?
(Multiple Choice)
4.9/5  (33)
(33)
The apparatus shown is called a closed-end manometer because the arm not connected to the gas sample is closed to the atmosphere and is under vacuum. 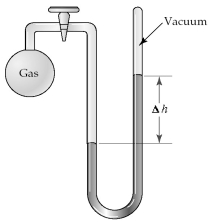 -When stopcock A of the open-end manometer shown below is opened,which drawing best represents the result?
-When stopcock A of the open-end manometer shown below is opened,which drawing best represents the result? 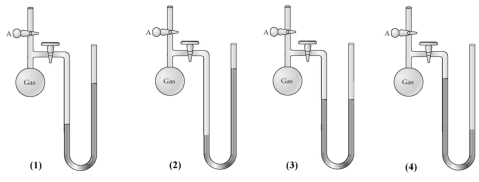
(Multiple Choice)
4.9/5  (31)
(31)
To two significant figures,by what factor will the pressure of an ideal gas change if the temperature of the gas is changed from 100°C to 200°C?
(Short Answer)
4.9/5  (41)
(41)
What is the pressure in a gas container that is connected to an open-end U-tube manometer if the pressure of the atmosphere is 722 torr and the level of mercury in the arm connected to the container is 8.60 cm higher than the level of mercury open to the atmosphere?
(Multiple Choice)
4.8/5  (33)
(33)
A 55.0-L steel tank at 20.0°C contains acetylene gas,C2H2,at a pressure of 1.39 atm.Assuming ideal behavior,how many grams of acetylene are in the tank?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following regions of the earth's atmosphere is closest to the surface of the earth?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 161 - 180 of 188
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)