Exam 10: Gases: Their Properties and Behavior
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Given three cylinders containing O2 gas at the same volume and pressure.Cylinder A is at -35°F,cylinder B is at -15°C,cylinder C is at 250 K.Which cylinder contains the largest mass of oxygen?
(Multiple Choice)
4.9/5  (39)
(39)
A 1.000 kg sample of nitroglycerine,C3H5N3O9,explodes and releases gases with a temperature of 1985°C at 1.400 atm.What is the volume of gas produced? 4 C3H5N3O9(s)→ 12 CO2(g)+ 10 H2O(g)+ 6 N2(g)+ O2(g)
(Multiple Choice)
4.9/5  (37)
(37)
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ne,cylinder B contains F2,and cylinder C contains Cl2.According to the kinetic molecular theory,which gas has the highest average speed?
(Multiple Choice)
4.8/5  (35)
(35)
At STP how many liters of NH3 can be produced from the reaction of 6.00 mol of N2 with 6.00 mol of H2? N2(g)+ 3 H2(g)→ 2 NH3(g)
(Multiple Choice)
4.8/5  (37)
(37)
A 0.429-g sample of gas occupies 125 mL at 60.cm of Hg and 25°C.What is the molar mass of the gas?
(Multiple Choice)
4.9/5  (29)
(29)
Assume that you have a mixture of nitrogen (molecular mass = 28 amu),represented by unshaded spheres,and chlorine (molecular mass = 71 amu),represented by shaded spheres at 300 K.Which of the drawings best represents the mixture? 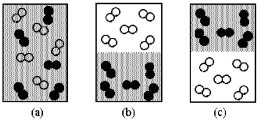
(Multiple Choice)
4.9/5  (35)
(35)
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1).The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2)-(4)most closely represents the result of doubling the number of moles of gas while keeping the pressure and temperature constant? 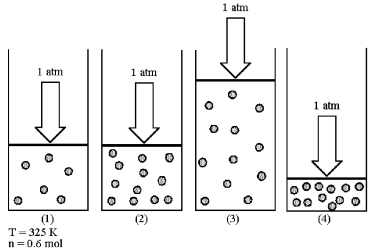
(Multiple Choice)
4.8/5  (35)
(35)
In the laboratory,hydrogen gas is usually made by the following reaction: Zn(s)+ 2 HCl(aq)→ H2(g)+ ZnCl2(aq)
How many liters of H2 gas,collected over water at an atmospheric pressure of 752 mm Hg and a temperature of 21.0°C,can be made from 3.132 g of Zn and excess HCl? The partial pressure of water vapor is 18.65 mm Hg at 21.0°C.
(Multiple Choice)
4.8/5  (41)
(41)
Showing 181 - 188 of 188
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)