Exam 10: Gases: Their Properties and Behavior
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
An approximation of absolute zero was made from an extrapolation of
(Multiple Choice)
4.8/5  (39)
(39)
A balloon contains 0.76 mol N2,0.18 mol O2,0.031 mol He and 0.026 mol H2 at 739 mm Hg.What is the partial pressure of O2?
(Multiple Choice)
4.8/5  (43)
(43)
An unknown gas effuses 2.3 times faster than N2O4 at the same temperature.What is the identity of the unknown gas?
(Multiple Choice)
4.9/5  (39)
(39)
How many liters of oxygen are needed to exactly react with 27.8 g of methane at STP? CH4(g)+ 2 O2(g)→ CO2(g)+ 2 H2O(l)
(Multiple Choice)
4.9/5  (34)
(34)
The volume of 350.mL of gas at 25°C is decreased to 250.mL at constant pressure.What is the final temperature of the gas?
(Multiple Choice)
4.8/5  (32)
(32)
If 0.40 mol of NaN3 reacts completely in the reaction shown below,then ________ L of N2 will be produced at STP.
2 NaN3(s)→ 2 Na(s)+ 3 N2(g)
(Short Answer)
4.8/5  (30)
(30)
Chloroform is a volatile liquid once commonly used in the laboratory but now being phased out due to its ozone depletion potential.If the pressure of gaseous chloroform in a flask is 195 mm Hg at 25°C and its density is 1.25 g/L,what is the molar mass of chloroform?
(Multiple Choice)
4.9/5  (40)
(40)
Suppose you needed to closely monitor small changes in pressure inside a container using an open end manometer.For the best accuracy,the substance in the manometer should
(Multiple Choice)
4.8/5  (40)
(40)
At an atmospheric pressure of 745 mm Hg,what is the pressure of He gas inside a cylinder that is attached to an open-end manometer in which the level of mercury in the open side of the manometer is 25 mm Hg higher than the side that is attached to the gas cylinder?
(Short Answer)
4.8/5  (32)
(32)
An unknown gas effuses 1.73 times faster than krypton.What is the molar mass of the gas?
(Multiple Choice)
4.9/5  (38)
(38)
A process by which gas molecules escape through a tiny hole in a membrane into a vacuum without collisions is called
(Multiple Choice)
4.8/5  (36)
(36)
Each of three identical 15.0-L gas cylinders contains 7.50 mol of gas at 295 K.Cylinder A contains Ar,cylinder B contains Cl2,and cylinder C contains N2.According to the kinetic molecular theory,which gas has the highest collision frequency?
(Multiple Choice)
4.9/5  (47)
(47)
A 1.75-L container filled with CO2 gas at 25°C and 225 kPa pressure springs a leak.When the container is re-sealed,the pressure is 185 kPA and the temperature is 10°C.How many moles of gas were lost?
(Multiple Choice)
4.8/5  (37)
(37)
The partial pressures of CH4,N2,and O2 in a sample of gas were found to be 143 mmHg,469 mmHg,and 251 mmHg,respectively.Calculate the mole fraction of oxygen.
(Multiple Choice)
4.8/5  (36)
(36)
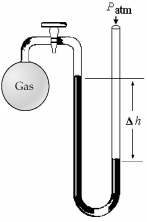 -What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 736 mm Hg and the difference in mercury levels,Δh,is 18 mm Hg?
-What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 736 mm Hg and the difference in mercury levels,Δh,is 18 mm Hg?
(Multiple Choice)
4.8/5  (35)
(35)
If mercury (density = 13.6 g/cm3)at a height of 745 mm Hg in a mercury barometer is replaced with water (density = 1.00 g/cm3),under the same conditions the height of water will be
(Multiple Choice)
4.9/5  (32)
(32)
Showing 61 - 80 of 188
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)