Exam 10: Gases: Their Properties and Behavior
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Two moles of neon gas at 20.0°C are heated to 350°C while the volume is kept constant.The density of the gas
(Multiple Choice)
4.8/5  (32)
(32)
The action of some commercial drain cleaners is based on the following reaction: 2 NaOH(s)+ 2 Al(s)+ 6 H2O(l)→ 2 NaAl(OH)4(s)+ 3 H2(g)
What is the volume of H2 gas formed at STP when 4.32 g of Al reacts with excess NaOH?
(Multiple Choice)
4.8/5  (35)
(35)
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1).The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2)-(4)most closely represents the result of doubling the temperature while keeping the pressure and number of moles of gas constant? 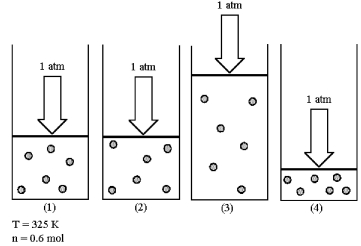
(Multiple Choice)
4.8/5  (32)
(32)
According to the kinetic molecular theory of gases,the volume of the gas particles (atoms or molecules)is ________ compared to the volume of the container in which the gas particles are held.
(Short Answer)
4.9/5  (29)
(29)
Three bulbs,two of which contain different gases and one of which is empty,are connected as shown in drawing (a).Which drawing (b)- (d)best represents the system after the stopcocks are opened and the system is allowed to come to equilibrium? 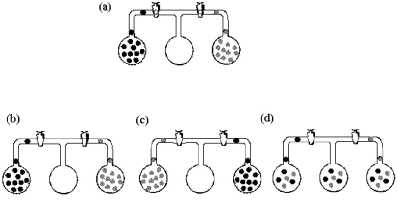
(Multiple Choice)
4.8/5  (44)
(44)
A gas occupies 22.4 L at STP and 16.5 L at 100°C and 1.75 atm pressure.How many moles of gas did the system gain or lose?
(Multiple Choice)
4.8/5  (39)
(39)
Cyanogen is a gas which contains 46.2% C and 53.8% N by mass.At a temperature of 25°C and a pressure of 750 mm Hg,1.50 g of cyanogen occupies 0.714 L.What is the molecular formula of cyanogen?
(Multiple Choice)
4.8/5  (41)
(41)
At what temperature will sulfur hexafluoride molecules have the same average speed as argon atoms at 20°C?
(Multiple Choice)
4.7/5  (34)
(34)
The apparatus shown is called a closed-end manometer because the arm not connected to the gas sample is closed to the atmosphere and is under vacuum. 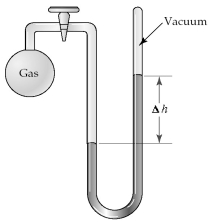 -What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 750 mm Hg and the difference in mercury levels,Δh,is 25 mm Hg?
-What is the pressure (in mm Hg)of the gas inside the above apparatus if the outside pressure,Patm,is 750 mm Hg and the difference in mercury levels,Δh,is 25 mm Hg?
(Multiple Choice)
4.9/5  (32)
(32)
What is the pressure in a gas container that is connected to an open-end U-tube manometer if the pressure of the atmosphere is 752 torr and the level of mercury in the arm connected to the container is 9.60 cm higher than the level of mercury open to the atmosphere?
(Multiple Choice)
4.8/5  (29)
(29)
What is the Celsius temperature of 100.0 g of chlorine gas in a 4.00-L container at 800 mm Hg?
(Multiple Choice)
4.9/5  (37)
(37)
What is the temperature of N2 gas if the average speed (actually the root-mean-square speed)of the molecules is 750 m/s?
(Multiple Choice)
4.8/5  (30)
(30)
If 1.0 mol of N2 and 3.0 mol of H2 in a closed container initially at STP react completely in the reaction shown below,then the final pressure in the flask will be ________ atm at 273 K.
N2(g)+ 3 H2(g)→ 2 NH3(g)
(Short Answer)
4.8/5  (36)
(36)
In a flask containing 2.00 mol of Ar,3.00 mol of HCN,and 5.00 mol of N2 at STP the partial pressure of He is ________ mm Hg.
(Short Answer)
4.9/5  (29)
(29)
A balloon contains 0.76 mol N2,0.18 mol Ar,0.031 mol He,and 0.026 mol H2 at 739 mm Hg.What is the partial pressure of Ar?
(Multiple Choice)
4.7/5  (40)
(40)
What is the total pressure in a 10.0 L flask which contains 0.200 mol of H2(g)and 0.215 mol of N2(g)at 20.0°C?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following gases has the highest average speed at 400K?
(Multiple Choice)
4.8/5  (32)
(32)
What is the average speed (actually the root-mean-square speed)of a xenon atom at 27°C?
(Multiple Choice)
4.7/5  (38)
(38)
A carbon dioxide monitoring product provides a reading of 202 ppm.Calculate the percent carbon dioxide by volume.
(Multiple Choice)
4.8/5  (42)
(42)
Showing 41 - 60 of 188
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)