Exam 10: Gases: Their Properties and Behavior
Exam 1: Chemical Tools: Experimentation and Measurement154 Questions
Exam 2: Atoms, molecules, and Ions275 Questions
Exam 3: Mass Relationships in Chemical Reactions159 Questions
Exam 4: Reactions in Aqueous Solutions211 Questions
Exam 5: Periodicity and the Electronic Structure of Atoms168 Questions
Exam 6: Ionic Compounds: Periodic Trends and Bonding138 Questions
Exam 7: Covalent Bonding and Electron-Dot Structures92 Questions
Exam 8: Covalent Compounds: Bonding Theories and Molecular Structure205 Questions
Exam 9: Thermochemistry: Chemical Energy170 Questions
Exam 10: Gases: Their Properties and Behavior188 Questions
Exam 11: Liquids, solids, and Phase Changes141 Questions
Exam 12: Solutions and Their Properties198 Questions
Exam 13: Chemical Kinetics190 Questions
Exam 14: Chemical Equilibrium171 Questions
Exam 15: Aqueous Equilibria: Acids and Bases231 Questions
Exam 16: Applications of Aqueous Equilibria201 Questions
Exam 17: Thermodynamics: Entropy, free Energy, and Equilibrium150 Questions
Exam 18: Electrochemistry212 Questions
Exam 19: Nuclear Chemistry176 Questions
Exam 20: Transition Elements and Coordination Chemistry190 Questions
Exam 21: Metals and Solid-State Materials154 Questions
Exam 22: The Main-Group Elements294 Questions
Exam 23: Connections to Organic and Biological Chemistry290 Questions
Select questions type
Some assumptions from the kinetic molecular theory are listed below.Which one is most frequently cited to explain Charles' law?
(Multiple Choice)
4.8/5  (44)
(44)
The burning of sulfur-containing coal can lead the formation of ________ which is emitted into the atmosphere.
(Short Answer)
4.8/5  (38)
(38)
The pressure in a container of gas connected to an open-end mercury manometer in which the mercury level is 22 cm lower in the side open to the atmosphere and the atmospheric pressure is 734 mm Hg is ________ mm Hg.
(Short Answer)
4.8/5  (35)
(35)
A container filled with gas is connected to an open-end manometer that is filled with mineral oil.The pressure in the gas container is 763 mm Hg and atmospheric pressure is 734 mm.How high will the level rise in the manometer if the densities of Hg and mineral oil are 13.6 g/mL and 0.822 g/mL respectively?
(Multiple Choice)
5.0/5  (38)
(38)
One reaction that contributes to photochemical smog is shown below.Which of the species involved contains one or more unpaired electrons? NO2(g)+ hυ →NO(g)+ O(g)
(Multiple Choice)
4.8/5  (36)
(36)
In the diagram below,helium atoms are represented by unshaded spheres,neon atoms by gray spheres,and argon atoms by black spheres.  -If the total pressure in the container is 900 mm Hg,what is the partial pressure of argon?
-If the total pressure in the container is 900 mm Hg,what is the partial pressure of argon?
(Multiple Choice)
5.0/5  (38)
(38)
When using the ideal gas law the temperature must be expressed in ________.
(Short Answer)
4.7/5  (34)
(34)
At STP if 1.00 mole of gas occupies 22.4 L then 0.200 mole of gas would occupy ________ L under the same conditions.
(Short Answer)
4.8/5  (39)
(39)
What is the temperature of SO2 gas if the average speed (actually the root-mean-square speed)of the molecules is 750 m/s?
(Multiple Choice)
4.8/5  (36)
(36)
A 2.000 kg sample of nitroglycerine,C3H5N3O9,explodes and releases gases with a temperature of 1985°C at 1.000 atm.What is the volume of gas produced? 4 C3H5N3O9(l)→ 12 CO2(g)+ 10 H2O(g)+ 6 N2(g)+ O2(g)
(Multiple Choice)
4.9/5  (39)
(39)
A glass tube has one end in a dish of mercury and the other end closed by a stopcock.The distance from the surface of the mercury to the bottom of the stopcock is 800 mm,as indicated by the meter stick shown in the drawing below.The apparatus is at 20°C,and the mercury level in the tube is the same as that in the dish. 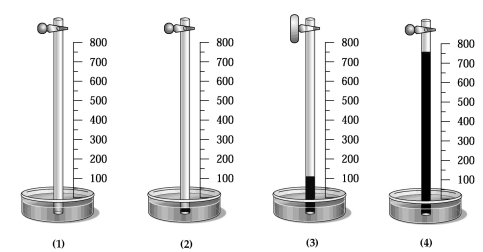 -Which drawing best shows the approximate level of the mercury in the tube when a vacuum pump is connected to the top of the tube,the stopcock opened,the tube is evacuated,the stopcock is closed,and the pump is removed,and the stopcock is reopened?
-Which drawing best shows the approximate level of the mercury in the tube when a vacuum pump is connected to the top of the tube,the stopcock opened,the tube is evacuated,the stopcock is closed,and the pump is removed,and the stopcock is reopened?
(Multiple Choice)
4.9/5  (38)
(38)
At STP the number of liters of O2 required to react with 11.2 liters of CH4 to form only CO2 and H2O is ________ liters.
(Short Answer)
4.8/5  (31)
(31)
Under the same pressure and temperature conditions,the level of water inside a barometer will be ________ than the level of mercury inside the barometer by a factor equal to the ratio of the density of ________ over the density of ________.
(Short Answer)
4.8/5  (31)
(31)
A 5.00-L flask contains nitrogen gas at 25°C and 1.00 atm pressure.What is the final pressure in the flask if an additional 2.00 g of N2 gas is added to the flask and the flask cooled to -55°C?
(Multiple Choice)
4.8/5  (34)
(34)
In the diagram below,helium atoms are represented by unshaded spheres,neon atoms by gray spheres,and argon atoms by black spheres.  -If the total pressure in the container is 900 mm Hg,what is the partial pressure of helium?
-If the total pressure in the container is 900 mm Hg,what is the partial pressure of helium?
(Multiple Choice)
4.7/5  (46)
(46)
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1).The initial pressure,number of moles,and temperature of the gas are noted on the diagram. 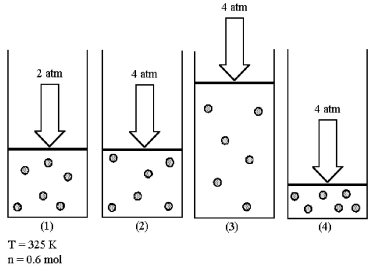 -Which diagram (2)-(4)most closely represents the result of doubling the pressure while keeping the temperature and number of moles of gas constant?
-Which diagram (2)-(4)most closely represents the result of doubling the pressure while keeping the temperature and number of moles of gas constant?
(Multiple Choice)
4.8/5  (32)
(32)
What is the volume of 20.0 g of argon gas at 157°C and 2.50 kPa pressure?
(Multiple Choice)
4.7/5  (39)
(39)
Which one of the following gases will have the lowest rate of effusion?
(Multiple Choice)
4.8/5  (33)
(33)
In the diagram below,helium atoms are represented by unshaded spheres,neon atoms by gray spheres,and argon atoms by black spheres.  -If the total pressure in the container is 900 mm Hg,what is the partial pressure of neon?
-If the total pressure in the container is 900 mm Hg,what is the partial pressure of neon?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 141 - 160 of 188
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)