Exam 1: Introduction: Matter, energy, and Measurement
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The recommended adult dose of Elixophyllin 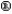 ,a drug used to treat asthma,is 6.00 mg/kg of body mass.Calculate the dose in milligrams for a 134-lb person.
,a drug used to treat asthma,is 6.00 mg/kg of body mass.Calculate the dose in milligrams for a 134-lb person.
(Multiple Choice)
4.9/5  (33)
(33)
If an object weighs 38.325 lbs,what would be the mass in grams?
(2.20 lbs = 1 kg)
(Short Answer)
4.7/5  (27)
(27)
There are ________ significant figures in the answer to the following computation: 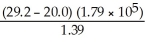
(Multiple Choice)
4.8/5  (43)
(43)
The density (in g/cm3)of a gold nugget that has a volume of 1.68 cm3 and a mass of 32.4 g is ________.
(Multiple Choice)
4.8/5  (32)
(32)
The correct result (indicating the proper number of significant figures)of the following calculation of the molecular mass for 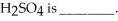 4 × 15.9994 + 32.066 + 2 × 1.0079
4 × 15.9994 + 32.066 + 2 × 1.0079
(Multiple Choice)
4.9/5  (34)
(34)
In the following list,only ________ is not an example of matter.
(Multiple Choice)
5.0/5  (33)
(33)
The correct answer (reported to the proper number of significant figures)to the following is ________. (2.01)(6.936)/ 12 = ________
(Multiple Choice)
4.8/5  (35)
(35)
Mass and volume are often referred to as ________ properties of substances.
(Short Answer)
4.8/5  (41)
(41)
________ significant figures should be retained in the result of the following calculation. 
(Multiple Choice)
4.9/5  (33)
(33)
The density of silver is 10.5 g/cm3.A piece of silver that occupies a volume of 42.5 cm3 would have a mass of ________ g.
(Multiple Choice)
4.8/5  (34)
(34)
A 210.lbs person is required to take a medication at a dose of 5.00 mg per kg of body weight twice a day.How much medication would the person take in a 24 hour period? (Indicate the number in proper scientific notation with the appropriate number of significant figures.)
(Multiple Choice)
4.9/5  (39)
(39)
A wooden object has a mass of 10.782 g and occupies a volume of 13.72 mL.What is the density of the object determined to an appropriate number of significant figures?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 141 - 160 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)