Exam 1: Introduction: Matter, energy, and Measurement
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
How many Significant Figures does the number 0.005000 have?
(Multiple Choice)
4.9/5  (34)
(34)
If an object is traveling at a speed of 400 m/s,how long (in hours)will it take to reach 950.5 km?
(Multiple Choice)
4.9/5  (27)
(27)
You have to calculate the volume of a gas sample with mass of 1.000 × 103 g and density of 1.027 g/L,but you have forgotten the formula.Which way of reasoning would help you in finding the correct mass?
(Multiple Choice)
4.8/5  (30)
(30)
What is the volume in cm3 of a perfect cube if one edge length measures 1.95 m?
(Multiple Choice)
4.8/5  (30)
(30)
The correct result (indicating the proper number of significant figures)of the following problem is ________. 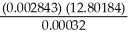
(Multiple Choice)
4.8/5  (40)
(40)
You have to calculate the mass of a 30.0 mL liquid sample with density of 1.52 g/mL,but you have forgotten the formula.Which way of reasoning would help you in finding the correct mass?
(Multiple Choice)
4.8/5  (35)
(35)
An object measuring 25.0 inches will have a length of ________ centimeters.
(Multiple Choice)
4.8/5  (40)
(40)
The density of lead is 11.4 g/cm3.The mass of a lead ball with a radius of 0.50 mm is ________ g. 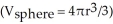
(Multiple Choice)
4.8/5  (27)
(27)
Osmium has a density of 22.6 g/cm3.What volume (in cm3)would be occupied by a 21.8 g sample of osmium?
(Multiple Choice)
4.9/5  (40)
(40)
The density of a 167.4 g sample of magnesium having a volume of 96.32 mL is ________ g/cm3.
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following is an illustration of the law of constant composition?
(Multiple Choice)
4.9/5  (37)
(37)
Which one of the following is often separated into its components by simple techniques such as filtering or decanting?
(Multiple Choice)
4.7/5  (35)
(35)
Which one of the following has the element name and symbol correctly matched?
(Multiple Choice)
5.0/5  (35)
(35)
A statement or mathematical equation which is based on repeated observations is called a(n)________.
(Multiple Choice)
4.9/5  (34)
(34)
Showing 41 - 60 of 163
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)