Exam 2: Atoms, molecules, and Ions
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Of the three types of radioactivity characterized by Rutherford,which type does not become deflected by a electric field?
(Multiple Choice)
4.8/5  (41)
(41)
When a bromine atom forms the bromide ion,it has the same charge as the ________ ion.
(Multiple Choice)
4.7/5  (33)
(33)
Barium reacts with a polyatomic ion to form a compound with the general formula Ba3(X)2.What would be the most likely formula for the compound formed between sodium and the polyatomic ion X?
(Multiple Choice)
4.8/5  (30)
(30)
Which element forms an ion with the same charge as the ammonium ion?
(Multiple Choice)
4.8/5  (31)
(31)
Which pair of elements would you expect to exhibit the greatest similarity in their physical and chemical properties?
(Multiple Choice)
4.8/5  (29)
(29)
The element X has three naturally occurring isotopes.The masses (amu)and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 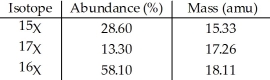
(Multiple Choice)
4.8/5  (33)
(33)
Horizontal rows of the periodic table are known as ________.
(Multiple Choice)
4.7/5  (39)
(39)
Different isotopes of a particular element contain different numbers of ________.
(Multiple Choice)
4.8/5  (42)
(42)
Showing 61 - 80 of 249
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)