Exam 6: Electronic Structure of Atoms
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
What is the maximum angular momentum quantum number in the ground state electron configuration of iodine?
(Multiple Choice)
4.7/5  (39)
(39)
Which one of the following is not a valid value for the magnetic quantum number of an electron in a 5d subshell?
(Multiple Choice)
4.7/5  (30)
(30)
Which of the quantum number(s)below represent the principal quantum number?
(Multiple Choice)
4.9/5  (32)
(32)
A 13 kg object is traveling at a velocity of 12.0 m/s.What is the de Broglie wavelength of this object?
(Multiple Choice)
4.7/5  (27)
(27)
The electron configuration of a ground-state Ag atom is ________.
(Multiple Choice)
4.9/5  (29)
(29)
All of the following are a result from the solution of the Schrodinger equation except ________.
(Multiple Choice)
4.8/5  (31)
(31)
There are ________ unpaired electrons in a ground state chlorine atom.
(Multiple Choice)
4.7/5  (34)
(34)
Of the following transitions in the Bohr hydrogen atom,the ________ transition results in the absorption of the highest-energy photon.
(Multiple Choice)
4.8/5  (39)
(39)
The larger the principal quantum number of an orbital,the lower is the energy of the electrons in that orbital.
(True/False)
4.9/5  (36)
(36)
The n = 2 to n = 6 transition in the Bohr hydrogen atom corresponds to the ________ of a photon with a wavelength of ________ nm.
(Multiple Choice)
4.9/5  (32)
(32)
Which of the subshells below do not exist due to the constraints upon the angular momentum quantum number?
(Multiple Choice)
4.8/5  (30)
(30)
Which orbital is degenerate with a 3dz2 in a many-electron atom?
(Multiple Choice)
4.8/5  (30)
(30)
The element that corresponds to the electron configuration 1s22s22p2 is ________.
(Multiple Choice)
4.7/5  (42)
(42)
The element that has a valence configuration of 5s25p6 is ________.
(Multiple Choice)
4.8/5  (37)
(37)
How many quantum numbers are necessary to designate a particular electron in an atom?
(Multiple Choice)
4.7/5  (30)
(30)
Using Bohr's equation for the energy levels of the electron in the hydrogen atom,determine the 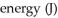 of an electron in the n = 4 level.
of an electron in the n = 4 level.
(Multiple Choice)
4.8/5  (34)
(34)
What is the wavelength of light (nm)that has a frequency 4.62 × 1014 s-1?
(Multiple Choice)
4.9/5  (28)
(28)
Showing 101 - 120 of 186
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)