Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension174 Questions
Exam 3: Vectors and Motion in Two Dimensions183 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity73 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work223 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids115 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics155 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces95 Questions
Exam 21: Electric Potential144 Questions
Exam 22: Current and Resistance125 Questions
Exam 23: Circuits157 Questions
Exam 24: Magnetic Fields and Forces168 Questions
Exam 25: EM Induction and Em Waves185 Questions
Exam 26: AC Electricity122 Questions
Exam 27: Relativity126 Questions
Exam 28: Quantum Physics86 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A sample of ideal monatomic gas is cooled by 50.0 C° at constant volume by removing 831 J of energy from it. How many moles of gas are in the sample? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (41)
(41)
An object having a fixed emissivity of 0.725 radiates heat at a rate of 10 W when it is at an absolute temperature T. If its temperature is doubled to 2T, at what rate will it now radiate?
(Multiple Choice)
4.8/5  (36)
(36)
Two metal spheres are made of the same material and have the same diameter, but one is solid and the other is hollow. If their temperature is increased by the same amount,
(Multiple Choice)
4.8/5  (46)
(46)
As shown in the figure, a bimetallic strip, consisting of metal G on the top and metal H on the bottom, is rigidly attached to a wall at the left. The coefficient of linear thermal expansion for metal G is greater than that of metal H. If the strip is uniformly heated, it will 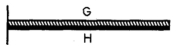
(Multiple Choice)
4.9/5  (25)
(25)
It is necessary to determine the specific heat of an unknown object. The mass of the object is It is determined experimentally that it takes to raise the temperature What is the specific heat of the object?
(Multiple Choice)
4.9/5  (28)
(28)
How much power does a sphere with a radius of 10 cm radiate into empty space if is has an emissivity of 1.0 and is kept at a temperature of 400 K? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.9/5  (40)
(40)
The figure shows a pV diagram for an ideal gas that is carried around a cyclic process. How much work is done in one cycle if p0 = and L? (1.00 atm = 101 kPa) 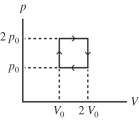
(Multiple Choice)
4.9/5  (43)
(43)
The coefficient of linear expansion of aluminum is 24.0 × 10-6 K-1, and the density of aluminum at 0°C is 2.70 × 103 kg/m3. What is the density of aluminum at 300°C?
(Multiple Choice)
4.8/5  (33)
(33)
A 771.0-kg copper bar is put into a smelter for melting. The initial temperature of the copper is 300.0 K. How much heat must the smelter produce to completely melt the copper bar? The specific heat for copper is 386 J/kg∙K, the heat of fusion for copper is 205,000 J/kg, and its melting point is 1357 K.
(Multiple Choice)
4.7/5  (42)
(42)
A machine part consists of 0.10 kg of iron (of specific heat 470 J/kg ∙ K ) and 0.16 kg of copper (of specific heat 390 J/kg ∙ K). How much heat must be added to the gear to raise its temperature from 18°C to 53°C?
(Multiple Choice)
5.0/5  (21)
(21)
A giant star radiates energy at the rate of 3.0 × 1030 W, and its surface temperature has been measured to be 3000 K. Assuming that it is a perfect emitter, what is the radius of this star?(σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.8/5  (25)
(25)
Two containers of equal volume each hold samples of the same ideal gas. Container A has twice as many molecules as container B. If the gas pressure is the same in the two containers, the correct statement regarding the absolute temperatures TA and TB in containers A and B, respectively, is
(Multiple Choice)
4.8/5  (27)
(27)
A 360-g metal container, insulated on the outside, holds 180.0 g of water in thermal equilibrium at 22.0°C. A 24.0-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.0°C. Assume there is no heat exchange with the surroundings. For water, the specific heat capacity is 4190 J/kg ∙ K and the heat of fusion is 3.34 × 105 J/kg. What is the specific heat capacity of the metal of the container?
(Multiple Choice)
4.9/5  (43)
(43)
A gas-filled vertical cylinder, closed at the bottom end, is fitted at the top with a piston that can move freely. The mass of the piston is 10.0 kg, and the initial height of the piston above the bottom of the cylinder is 25 cm. A mass of 8.0 kg is placed on the piston. What is the resulting height of the piston, assuming that the temperature of the ideal gas is kept constant?
(Multiple Choice)
4.9/5  (41)
(41)
If an ideal gas molecule has a speed of 0.50 km/s at 20°C, what is its speed at 80°C?
(Multiple Choice)
4.8/5  (28)
(28)
Consider a flat steel plate with a hole through its center as shown in the figure. When the temperature of the plate is increased, the hole will 
(Multiple Choice)
4.9/5  (27)
(27)
Consider two equal-volume flasks of gas at the same temperature and pressure. One gas, oxygen, has a molecular mass of 32. The other gas, nitrogen, has a molecular mass of 28. What is the ratio of the number of oxygen molecules to the number of nitrogen molecules in these flasks?
(Multiple Choice)
4.9/5  (40)
(40)
The melting point of aluminum is 660°C, its latent heat of fusion is 4.00 × 105 J/kg, and its specific heat is 900 J/kg ∙ K. If 300 kJ of heat are added to 442 g of aluminum at 100°C, what is the final state of the system? That is, how much is liquid, how much is solid, and what is its temperature?
(Essay)
4.9/5  (35)
(35)
A glass beaker of unknown mass contains of water. The system absorbs of heat and the temperature rises as a result. What is the mass of the beaker? The specific heat of glass is 0.18 cal/g ∙ °C, and that of water is 1.0 cal/g ∙ C°.
(Multiple Choice)
5.0/5  (28)
(28)
The figure shows a pV diagram for 0.0077 mol of ideal gas that undergoes the process 1 → 2 → 3. What is the volume V3? (R = 8.31 J/mol ∙ K) 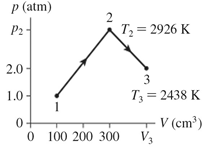 the volume V3?
the volume V3?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 181 - 200 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)