Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension174 Questions
Exam 3: Vectors and Motion in Two Dimensions183 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity73 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work223 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids115 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics155 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces95 Questions
Exam 21: Electric Potential144 Questions
Exam 22: Current and Resistance125 Questions
Exam 23: Circuits157 Questions
Exam 24: Magnetic Fields and Forces168 Questions
Exam 25: EM Induction and Em Waves185 Questions
Exam 26: AC Electricity122 Questions
Exam 27: Relativity126 Questions
Exam 28: Quantum Physics86 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A 0.600-kg piece of metal X is heated to 100°C and placed in an aluminum can of mass 0.200-kg which contains 0.500 kg of water initially at 17.3°C. The final equilibrium temperature of the mixture is 20.2°C, what is the specific heat of metal X? The specific heats of water and aluminum are 4186 J/kg ∙ K (water) and 910 J/kg ∙ K (aluminum).
(Multiple Choice)
4.9/5  (31)
(31)
An ideal gas is held in a container of volume V at pressure p. The rms speed of a gas molecule under these conditions is v. If now the volume and pressure are changed to 2V and 2p, the rms speed of a molecule will be
(Multiple Choice)
5.0/5  (29)
(29)
A certain automobile tire has a volume of 0.0185 m3. If the absolute (or total) pressure in the tire is 500 kPa and the temperature is 298 K, how many molecules are there inside the tire? (R = 8.31 J/mol ∙ K, NA = 6.022 x 1023 molecules/mol)
(Multiple Choice)
4.8/5  (33)
(33)
In an electric furnace used for refining steel, the temperature is monitored by measuring the radiant power emitted through a small hole in the wall of the furnace, of area 0.5 cm2. This hole acts like a perfect blackbody radiator having the same temperature as the interior of the furnace. If the temperature of the furnace (and therefore of the hole) is to be maintained at 1650°C, how much power will the hole radiate?
(Multiple Choice)
4.7/5  (32)
(32)
The absolute temperature of an ideal gas is directly proportional to which of the following quantities?
(Multiple Choice)
4.9/5  (34)
(34)
A person makes iced tea by adding ice to 1.8 kg of hot tea, initially at 80°C. How many kilograms of ice, initially at 0°C, are required to bring the mixture to 10°C? The specific heat of water (and tea) is 4186 J/kg ∙ K, and the latent heat of fusion of ice is 3.34 × 105 J/kg.
(Multiple Choice)
4.7/5  (32)
(32)
How much heat must be added to a 8.0-kg block of ice at -8°C to change it to water at The specific heat of ice is 2050 J/kg ∙ C°, the specific heat of water is 4186 J/kg ∙ C°, the latent heat of fusion of ice is 334,000 J/kg, and 1 cal = 4.186 J.
(Multiple Choice)
4.8/5  (37)
(37)
Originally 2.00 mol of gas are at STP. If the temperature changes to 47.0°C and the pressure decreases to half of what it was, how many liters do the two moles now occupy? (1 atm = 101 kPa, R = 8.31 J/mol ∙ K)
(Short Answer)
4.9/5  (24)
(24)
(a) At what Celsius temperature is the average kinetic energy of a helium gas atom equal to 6.21 × 10-21 J? The Boltzmann constant is 1.38 × 10-23 J/K .
(b) What would be the temperature for radon gas?
(Short Answer)
4.9/5  (29)
(29)
When a sample of water at 0.0°C is cooled to -36.0°C and freezes in the process, 935,000 kJ of heat is liberated. What is the mass of this sample of water? For water LF = 334,000 J/kg, LV = 2.256 × 106 J/kg, and the specific heat of ice is 2050 J/kg ∙ C°.
(Multiple Choice)
4.7/5  (38)
(38)
Which one of the following quantities is the smallest unit of heat energy?
(Multiple Choice)
4.8/5  (33)
(33)
A blacksmith is flattening a steel plate having dimensions 10 cm × 15 cm × 1 mm. He has heated the plate to 900 K. If the emissivity of the plate is 0.75, at what rate does it lose energy by radiation? Ignore any heat exchange with the surroundings. (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.8/5  (34)
(34)
A 40.0-g block of ice at -15.00°C is dropped into a calorimeter (of negligible heat capacity) containing water at 15.00°C. When equilibrium is reached, the final temperature is 8.00°C. How much water did the calorimeter contain initially? The specific heat of ice is 2090 J/kg ∙ K, that of water is 4186 J/kg ∙ K, and the latent heat of fusion of water is 33.5 × 104 J/kg.
(Multiple Choice)
4.8/5  (40)
(40)
A solid concrete wall has dimensions 4.0 m × 2.4 m and is 30 cm thick. The thermal conductivity of the concrete is 1.3 W/m ∙ K, and it separates a basement from the ground outside. The inner surface of the wall is at 18°C, and the outside surface is at 6°C. How much heat flows through the wall every hour?
(Multiple Choice)
4.9/5  (35)
(35)
The coefficient of linear expansion of steel is 12 × 10-6 K-1. What is the change in length of a 25-m steel bridge span when it undergoes a temperature change of 40 K from winter to summer?
(Multiple Choice)
4.9/5  (32)
(32)
The coefficient of linear expansion of copper is 17 × 10-6 K-1. A sheet of copper has a round hole with a radius of 3.0 m cut out of it. If the sheet is heated and undergoes a change in temperature of 80 K, what is the change in the radius of the hole?
(Multiple Choice)
4.9/5  (32)
(32)
How much heat must be removed from 456 g of water at 25.0°C to change it into ice at -10.0°C? The specific heat of ice is 2090 J/kg ∙ K, the latent heat of fusion of water is 33.5 × 104 J/kg, and the specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (36)
(36)
An ideal gas undergoes the process a→b→c→a shown in the pV diagram. In this figure, Pa = Pc = 3.60 × 105 Pa, Vb = Vc = 68.00 L, Va = 35 L, and Pb = 5.60 × 105 Pa. How much work is done by the system in this process? 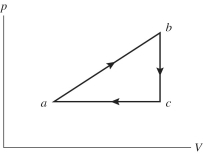
(Multiple Choice)
4.9/5  (33)
(33)
A container of 114.0 g of water is heated using of power, with perfect efficiency. How long will it take to raise the temperature of the water from to The specific heat capacity of the container is negligible, and the specific heat capacity of water is 4.186 × 103 J/kg ∙ C.
(Multiple Choice)
4.9/5  (33)
(33)
When 50 g of a certain material at 100°C is mixed with 100 g of water at 0°C, the final temperature is 40°C. What is the specific heat of the material? The specific heat of water is 1.00 kcal/kg ∙ C°.
(Multiple Choice)
4.9/5  (32)
(32)
Showing 141 - 160 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)