Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension174 Questions
Exam 3: Vectors and Motion in Two Dimensions183 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity73 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work223 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids115 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics155 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces95 Questions
Exam 21: Electric Potential144 Questions
Exam 22: Current and Resistance125 Questions
Exam 23: Circuits157 Questions
Exam 24: Magnetic Fields and Forces168 Questions
Exam 25: EM Induction and Em Waves185 Questions
Exam 26: AC Electricity122 Questions
Exam 27: Relativity126 Questions
Exam 28: Quantum Physics86 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A gas is taken through the cycle shown in the pV diagram in the figure. During one cycle, how much work is done by the gas? 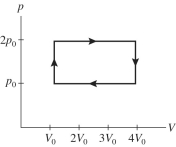
(Multiple Choice)
4.8/5  (34)
(34)
The coefficient of linear expansion of copper is 17 × 10-6 K-1. A block of copper 30 cm wide, 45 cm long, and 10 cm thick is heated from 0°C to 100°C What is the change in the volume of the block?
(Multiple Choice)
4.8/5  (40)
(40)
An oxygen molecule, O2, falls in a vacuum. From what height must it fall so that its translational kinetic energy at the bottom of its fall equals the average translational kinetic energy of an oxygen molecule at 920 K? The mass of one O2 molecule is 5.312 × 10-26 kg, and the Boltzmann constant is 1.38 × 10-23 J/K. Neglect air resistance and assume that g remains constant at 9.8 m/s2 throughout the fall of the molecule.
(Multiple Choice)
4.8/5  (29)
(29)
A concrete wall of a cold storage room measures 3.0 m high, 5.0 m wide, and 20 cm thick. The inside wall is to be covered by a layer of wood in order to reduce the rate of heat flow through the wall by 90 percent. The inner surface of the wooden wall is maintained at -10°C and the outer surface of the concrete wall is at 20°C. The thermal conductivities of concrete and wood are 0.80 W/m ∙ K (concrete) and 0.040 W/m ∙ K (wood). What is the temperature difference across the layer of wood?
(Multiple Choice)
4.7/5  (45)
(45)
A sample of an ideal gas is heated and its Kelvin temperature doubles. If the root-mean-square speed of its molecules was originally v, what is the new root-mean-square speed?
(Multiple Choice)
4.8/5  (32)
(32)
A steel pipe 36.0 m long, installed when the temperature was is used to transport superheated steam at a temperature of Steel's coefficient of linear expansion is . The pipe is allowed to expand freely when the steam is transported. What is the increase in the length of the pipe when it is used with the superheated steam?
(Multiple Choice)
4.9/5  (43)
(43)
Two metal rods, one silver and the other gold, are attached to each other end-to-end. The free end of the silver rod is immersed in a steam chamber at 100°C, and the free end of the gold rod in an ice water bath at 0°C. The rods are both 5.0 cm long and have a square cross-section that is 2.0 cm on a side. No heat is exchanged between the rods and their surroundings, except at the ends. How much total heat flows through the two rods each minute? The thermal conductivity of silver is 417 W/m ∙ K, and that of gold is 291 W/m ∙ K.
(Multiple Choice)
4.8/5  (29)
(29)
A 24.0-L tank contains ideal helium gas at 27°C and a pressure of 22.0 atm. How many moles of gas are in the tank? (R = 8.31 J/mol ∙ K, 1 atm = 101 kPa)
(Multiple Choice)
4.9/5  (38)
(38)
A 35-g block of ice at -14°C is dropped into a calorimeter (of negligible heat capacity) containing 400 g of water at 0°C. When the system reaches equilibrium, how much ice is left in the calorimeter? The specific heat of ice is 2090 J/kg ∙ K, that of water is 4186 J/kg ∙ K, and the latent heat of fusion of water is 33.5 × 104 J/kg.
(Multiple Choice)
4.7/5  (37)
(37)
A 200-L electric water heater uses 2.0 kW. Assuming no heat loss, how many hours would it take to heat the water in this tank from 23°C to 75°C? The specific heat of water is 4186 J/kg ∙ K and its density is 1000 kg/m3.
(Multiple Choice)
4.9/5  (36)
(36)
An ideal gas has a pressure of 2.5 atm, a volume of 1.0 L at a temperature of 30°C. How many molecules are there in this gas? (R = 8.31 J/mol ∙ K,1.00 atm = 101 kPa, NA = 6.022 × 1023)
(Multiple Choice)
5.0/5  (41)
(41)
A substance has a melting point of 20°C and a heat of fusion of 3.4 × J/kg. The boiling point is and the heat of vaporization is at a pressure of one atmosphere. The specific heats for the solid, liquid, and gaseous phases are 600 J/kg ∙ K (solid), 1000 J/kg ∙ K (liquid), and 400 J/kg ∙ K (gaseous). How much heat is required to raise the temperature of of this substance from to at a pressure of one atmosphere?
(Multiple Choice)
4.9/5  (28)
(28)
For the mercury in a thermometer to expand from 4.00 cm3 to 4.10 cm3, what change in temperature is necessary? The mercury has a volume expansion coefficient of 1.80 × 10-4 K-1.
(Multiple Choice)
4.9/5  (29)
(29)
A substance has a melting point of 20°C and a heat of fusion of The boiling point is and the heat of vaporization is at a pressure of one atmosphere. The specific heats for the solid, liquid, and gaseous phases are 600 J/kg ∙ K (solid), 1000 J/kg ∙ K (liquid), and 400 J/kg ∙ K (gaseous). How much heat is given up by of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?
(Multiple Choice)
4.8/5  (36)
(36)
How much heat is required to raise the temperature of a 225-g lead ball from 15.0°C to 25.0°C? The specific heat of lead is 128 J/kg ∙ K.
(Multiple Choice)
4.8/5  (36)
(36)
Your lungs hold 4.2 L of air at a temperature of 27°C and a pressure of 101.3 kPa. How many moles of air do your lungs hold? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.7/5  (30)
(30)
If we add 700 J of heat to 12 moles of an ideal monatomic gas at constant volume, what will be the change in temperature of the gas? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
5.0/5  (37)
(37)
A container with rigid walls is filled with 4.00 mol of air at 17°C with CV = 2.5R. What is the final temperature of the air if its internal energy is increased by 28 kJ? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (40)
(40)
A window glass that is 0.5 cm thick has dimensions of 3 m by 1.5 m. The thermal conductivity of this glass is 0.8 W/m ∙ K. If the outside surface of the glass is at -10°C and the inside surface is at 20°C, how much heat flows through the window in every hour?
(Multiple Choice)
4.9/5  (38)
(38)
A 600-g piece of iron at 100°C is dropped into a calorimeter of negligible heat capacity containing 100 g of ice at 0°C and 120 g of water, also at 0°C. What is the final temperature of the system? The specific heat of iron is 448 J/kg ∙ K, the latent heat of fusion of water is 33.5 × 104 J/kg, and the specific heat of water is 4186 J/kg ∙ K.
(Short Answer)
4.8/5  (37)
(37)
Showing 21 - 40 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)