Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension174 Questions
Exam 3: Vectors and Motion in Two Dimensions183 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity73 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work223 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids115 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics155 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces95 Questions
Exam 21: Electric Potential144 Questions
Exam 22: Current and Resistance125 Questions
Exam 23: Circuits157 Questions
Exam 24: Magnetic Fields and Forces168 Questions
Exam 25: EM Induction and Em Waves185 Questions
Exam 26: AC Electricity122 Questions
Exam 27: Relativity126 Questions
Exam 28: Quantum Physics86 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A beaker of negligible heat capacity contains 456 g of ice at -25.0°C. A lab technician begins to supply heat to the container at the rate of 1000 J/min. How long after starting will the ice begin to melt, assuming all of the ice has the same temperature? The specific heat of ice is 2090 J/kg ∙ K and the latent heat of fusion of water is 33.5 × 104 J/kg.
(Short Answer)
4.8/5  (27)
(27)
A 400-g block of iron at 400°C is dropped into a calorimeter (of negligible heat capacity) containing 60 g of water at 30°C. How much steam is produced? The latent heat of vaporization of water is 22.6 × 105 J/kg and its specific heat capacity is 4186 J/kg ∙ K. The average specific heat of iron over this temperature range is 560 J/kg ∙ K.
(Multiple Choice)
4.9/5  (36)
(36)
A mercury thermometer has a glass bulb of interior volume 0.100 cm3 at 10°C. The glass capillary tube above the bulb has an inner cross-sectional area of 0.012 mm2. The coefficient of volume expansion of mercury is 1.8 × 10-4 K-1. If the expansion of the glass is negligible, how much will the mercury rise in the capillary tube when the temperature rises from 5°C to 35°C if the bulb was full at 5°C?
(Multiple Choice)
4.9/5  (30)
(30)
The volume coefficient of thermal expansion for gasoline is 950 × 10-6 K-1. By how many cubic centimeters does the volume of 1.00 L of gasoline change when the temperature rises from 30°C to 50°C?
(Multiple Choice)
4.9/5  (41)
(41)
By what primary heat transfer mechanism does the sun warm the earth?
(Multiple Choice)
4.8/5  (37)
(37)
A refrigerator has an interior volume of 0.500 m3. The temperature inside the refrigerator in 282 K, and the pressure is 101 kPa. If the molecular weight of air is 29 g/mol, what is the mass of air inside the refrigerator? (R = 8.31 J/mol × K)
(Multiple Choice)
4.9/5  (30)
(30)
At what temperature would the root-mean-square speed of hydrogen, H2, molecules equal 11.2 km/s (the earth's escape speed)? The mass of a hydrogen atom is 1.67 × 10-27 kg, and the Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.8/5  (31)
(31)
The figure shows a pV diagram for 2.6 g of ideal helium gas that undergoes the process 1 → 2 → 3. Find the value of volume V3. The atomic mass of helium is 4.0 g/mol, and R = 8.31 J/mol ∙ K. 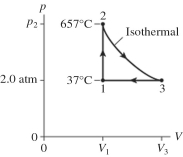
(Multiple Choice)
4.8/5  (33)
(33)
A heat-conducting rod that is wrapped in insulation is constructed with a 0.15-m length of alloy A and a 0.40-m length of alloy B, joined end-to-end. Both pieces have cross-sectional areas of 0.0020 m2. The thermal conductivity of alloy B is known to be 1.8 times as great as that for alloy A. The end of the rod in alloy A is maintained at a temperature of 10°C, and the other end of the rod is maintained at an unknown temperature. When steady state flow has been established, the temperature at the junction of the alloys is measured to be 40° C, and the rate of heat flow in the rod is measured at 56 W. What is the thermal conductivity of alloy A?
(Multiple Choice)
4.9/5  (36)
(36)
Two metal rods, one silver and the other gold, are attached to each other end-to-end. The free end of the silver rod is immersed in a steam chamber at 100°C, and the free end of the gold rod in an ice water bath at 0°C. The rods are both 5.0 cm long and have a square cross-section that is 2.0 cm on a side. No heat is exchanged between the rods and their surroundings, except at the ends. What is the temperature at the point where the two rods are in contact with one another? The thermal conductivity of silver is 417 W/m ∙ K, and that of gold is 291 W/m ∙ K.
(Multiple Choice)
4.8/5  (35)
(35)
If you add 700 kJ of heat to 700 g of water originally at 70.0°C, how much water is left in the container? The latent heat of vaporization of water is 22.6 × J/kg, and its specific heat capacity is 4186 J/kg ∙ K.
(Multiple Choice)
4.7/5  (27)
(27)
The rms speed of a certain sample of carbon dioxide molecules, with a molecular weight of 44.0 g/mole, is 396 m/s. What is the rms speed of water vapor molecules, with a molecular weight of 18.0 g/mol, at the same temperature as the carbon dioxide?
(Multiple Choice)
4.8/5  (25)
(25)
The figure shows a pV diagram for 0.0061 mol of ideal gas that undergoes the process 1 → 2 → 3. What is the pressure p2? (R = 8.31 J/mol ∙ K) 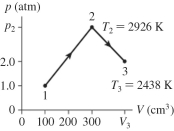
(Multiple Choice)
5.0/5  (39)
(39)
A person is walking outdoors on a cold day when the temperature is -20°C. He is breathing at the rate of 16 breaths per minute, and each time he breathes in he inhales 0.0050 m3 of air. At what rate does he lose heat from breathing if the air in his lungs is heated to body temperature (37°C) before it is exhaled? The specific heat of air is 1020 J/kg ∙ K and the density of air is 1.29 kg/m3.
(Multiple Choice)
4.7/5  (39)
(39)
A fixed container holds oxygen and helium gases at the same temperature. Which of the following statements are correct? (There could be more than one correct choice.)
(Multiple Choice)
4.9/5  (38)
(38)
On a cold day, a piece of metal feels much colder to the touch than a piece of wood. This is due to the difference in which one of the following physical properties of these materials?
(Multiple Choice)
4.8/5  (41)
(41)
A person tries to heat up her bath water by adding 5.0 L of water at 80°C to 60 L of water at 30°C. What is the final temperature of the bath water?
(Multiple Choice)
4.8/5  (38)
(38)
A jogger is running outdoors on a cold day when the temperature is -20.0°C. She is breathing at the rate of 25 breaths per minute, and each time she breathes in she inhales 0.00450 m3 of air. How much heat does she lose from breathing during 20.0 minutes of jogging if the air in her lungs is heated to her body temperature of 37.0°C before it is exhaled? The specific heat of air is 1020 J/kg ∙ K and the density of air under typical conditions is 1.29 kg/m3.
(Multiple Choice)
4.8/5  (36)
(36)
Dust particles in a grain elevator frequently have masses of the order of 1.0 × 10-9 kg. If, to a first approximation, we model the dust particles as an ideal gas, what would be the rms speed of such a particle in air at 27°C? The Boltzmann constant is 1.38 × 10-23 J/K .
(Multiple Choice)
4.8/5  (33)
(33)
At what temperature would the root mean square speed of oxygen molecules, O2, be if oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg, and the Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.8/5  (41)
(41)
Showing 161 - 180 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)