Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension174 Questions
Exam 3: Vectors and Motion in Two Dimensions183 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity73 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work223 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids115 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics155 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces95 Questions
Exam 21: Electric Potential144 Questions
Exam 22: Current and Resistance125 Questions
Exam 23: Circuits157 Questions
Exam 24: Magnetic Fields and Forces168 Questions
Exam 25: EM Induction and Em Waves185 Questions
Exam 26: AC Electricity122 Questions
Exam 27: Relativity126 Questions
Exam 28: Quantum Physics86 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A 6.5-g iron meteor hits the earth at a speed of 295 m/s. If its kinetic energy is entirely converted to heat in the meteor, by how much will its temperature rise? The specific heat of iron is 113 cal/kg ∙ C°, and 1 cal = 4.186 J.
(Multiple Choice)
4.9/5  (33)
(33)
An expansion process on an ideal diatomic ideal gas has a linear path between the initial and final coordinates on a pV diagram. The coordinates of the initial state are: the pressure is 300 kPa, the volume is and the temperature is The final pressure is and the final temperature is How much work is done by the gas during this process? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (35)
(35)
A rigid container is filled with 4.0 mol of air with CV = 2.5R. How much does the internal (thermal) energy of the air change if its temperature rises from to (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (31)
(31)
A vertical cylinder, closed at the bottom end, contains 0.0100 mol of ideal gas. It is fitted at the top with a piston that can move freely. The mass of the piston is 14.0 kg and the initial height of the piston above the bottom of the cylinder is 25 cm. What is the temperature of the gas? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (36)
(36)
The melting point of aluminum is 660°C, its latent heat of fusion is 4.00 × 105 J/kg, and its specific heat is 900J/kg ∙ K. How much heat must be added to 500 g of aluminum originally at 27°C to completely melt it?
(Multiple Choice)
5.0/5  (34)
(34)
A car starts out when the air temperature is 288 K and the absolute (total) air pressure in the tires is 500 kPa. After driving a while, the temperature of the air in the tires increases to 298 K. What is the pressure in the tires at that point, assuming their volume does not change?
(Multiple Choice)
4.9/5  (37)
(37)
A heat-conducting rod, 0.90 m long and wrapped in insulation, is made of an aluminum section that is 0.20 m long and a copper section that is long. Both sections have a cross-sectional area of The aluminum end and the copper end are maintained at temperatures of and respectively. The thermal conductivities of aluminum and copper are 205 W/m ∙ K (aluminum) and 385 W/m ∙ K (copper). What is the temperature of the aluminum-copper junction in the rod with steady state heat flow?
(Multiple Choice)
4.7/5  (39)
(39)
A 2294-kg sample of water at 0° C is cooled to and freezes in the process. How much heat is liberated? For water LF = 334,000 J/kg and LV = 2.256 × 106 J/kg. The specific heat of ice is 2050 J/kg ∙ K.
(Multiple Choice)
4.7/5  (36)
(36)
A 3.9-L volume of ideal neon gas (monatomic) is at a pressure of 5.6 aym and a temperature of The atomic mass of neon is The temperature of the gas is now increased to 430 K and the volume is increased to What is the final pressure of the gas?
(Multiple Choice)
4.9/5  (31)
(31)
What is the total translational kinetic energy of the gas in a classroom filled with nitrogen at at The dimensions of the classroom are The Boltzmann constant is 1.3806503 × 10-23 J/K, R = 8.31 J/mol ∙ K, and NA = 6.022 × 1023 molecules/mol.
(Short Answer)
4.9/5  (40)
(40)
Suppose that a rigid aluminum wire were to be strung out in a loop that just fits snugly around the equator (assuming a perfectly spherical Earth with a radius of 6.37 × 106 m). If the temperature of the wire is increased by 0.50°C, and the increase in length is distributed equally over the entire length, how far off the ground will the wire loop be if it remained centered on the earth? The coefficient of linear expansion of aluminum is 24 × 10-6 K-1.
(Multiple Choice)
5.0/5  (39)
(39)
A certain ideal gas has a molar specific heat at constant pressure of 33.2 J/mol ∙ K. Its molar specific heat at constant volume is closest to which of the following values? (R = 8.31J/mol ∙ K)
(Multiple Choice)
4.9/5  (38)
(38)
How many molecules are in (a) 1.0 cm3 of air at STP and (b) 1.0 cm3 of helium at STP? (R = 8.31 J/mol . K, NA = 6.022 × 1023 molecules/mol)
(Short Answer)
4.8/5  (37)
(37)
A balloon originally has a volume of 1.0 m3 when the gas in it is at 20°C and under a pressure of 1.0 atm. As it rises in the earth's atmosphere, its volume expands. What will be its new volume if its final temperature and pressure are -40°C and 0.10 atm?
(Multiple Choice)
4.9/5  (33)
(33)
Two processes are shown on the pV diagram in the figure. One of them is an adiabat and the other one is an isotherm. Which process is the isotherm? 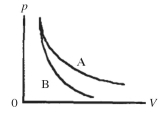
(Multiple Choice)
4.9/5  (36)
(36)
A sealed container holds 0.020 moles of ideal nitrogen (N2) gas, at a pressure of 1.5 atm and a temperature of 290 K. The atomic mass of nitrogen is 14.0 g/mol. What is the average translational kinetic energy of a nitrogen molecule? The Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.8/5  (34)
(34)
Two experimental runs are performed to determine the calorimetric properties of an alcohol which has a melting point of -10.0° C. In the first run, a 200-g cube of frozen alcohol, at the melting point, is added to 300 g of water at 20.0°C in a styrofoam container. When thermal equilibrium is reached, the alcohol-water solution is at a temperature of 5.0°C. In the second run, an identical cube of alcohol is added to 500 g of water at 20.0°C and the temperature at thermal equilibrium is 10.0°C. The specific heat capacity of water is 4190 J/kg ∙ K. Assume no heat is exchanged with the styrofoam container and the surroundings. What is the heat of fusion of the alcohol?
(Multiple Choice)
4.8/5  (41)
(41)
How much heat is required to raise the temperature of 2.00 moles of an ideal monatomic gas by 10 C° at constant volume? (R = 8.31 J/mol ∙ K).
(Multiple Choice)
4.9/5  (41)
(41)
A weather balloon containing 2.0 m3 of hydrogen gas rises from a location at which the temperature is 22°C and the pressure is 101 kPa to a location where the temperature is -39°C and the pressure is 20 kPa. If the balloon is free to expand so that the pressure of the gas inside is equal to the ambient pressure, what is the new volume of the balloon?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 101 - 120 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)