Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension174 Questions
Exam 3: Vectors and Motion in Two Dimensions183 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity73 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work223 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids115 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics155 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces95 Questions
Exam 21: Electric Potential144 Questions
Exam 22: Current and Resistance125 Questions
Exam 23: Circuits157 Questions
Exam 24: Magnetic Fields and Forces168 Questions
Exam 25: EM Induction and Em Waves185 Questions
Exam 26: AC Electricity122 Questions
Exam 27: Relativity126 Questions
Exam 28: Quantum Physics86 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A solid cylindrical bar conducts heat at a rate of 25 W from a hot to a cold reservoir under steady state conditions. If both the length and the diameter of this bar are doubled, the rate at which it will conduct heat between these reservoirs will be
(Multiple Choice)
4.9/5  (26)
(26)
The radius of a star is 6.95 × 108 m, and its rate of radiation has been measured to be 5.32 × 1026 W. Assuming that it is a perfect emitter, what is the temperature of the surface of this star? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.9/5  (31)
(31)
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper, with the aluminum and the copper in thermal contact. The specific heat capacity of aluminum is more than double that of copper. Which object experiences the greater magnitude gain or loss of heat during the time the system takes to reach thermal equilibrium?
(Multiple Choice)
4.8/5  (26)
(26)
The thermal conductivity of a certain concrete is 0.80 W/m . K and the thermal conductivity of a certain wood is 0.10 W/m ∙ K. How thick would a solid concrete wall have to be in order to have the same rate of heat flow through it as an 8.0-cm thick wall made of solid wood? Both walls have the same surface area and the same temperature difference across their faces.
(Multiple Choice)
4.8/5  (35)
(35)
What is the average translational kinetic energy of an ideal gas at The Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.9/5  (26)
(26)
An 920-g piece of iron at 100°C is dropped into a calorimeter of negligible heat capacity containing 50 g of ice at 0°C and 92 g of water, also at 0°C. What is the final temperature of the system? The specific heat of iron is 448 J/kg ∙ K, that of water is 4186 J/kg ∙ K, and the latent heat of fusion of water is 33.5 × 104 J/kg.
(Multiple Choice)
4.9/5  (43)
(43)
The water flowing over Niagara Falls drops a distance of 50 m. If all the gravitational potential energy is converted to thermal energy, by what temperature does the water rise? The specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.9/5  (40)
(40)
How many grams of ice at -17°C must be added to 741 grams of water that is initially at a temperature of to produce water at a final temperature of Assume that no heat is lost to the surroundings and that the container has negligible mass. The specific heat of liquid water is 4190 J/kg ∙ C° and of ice is 2000 J/kg ∙ C°. For water the normal melting point is 0°C and the heat of fusion is 334 × 103 J/kg. The normal boiling point is 100°C and the heat of vaporization is 2.256 × 106 J/kg.
(Short Answer)
4.8/5  (36)
(36)
The figure shows a pV diagram for 0.98 mol of ideal gas that undergoes the process 1 → 2. The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? (R = 8.31 J/mol ∙ K). 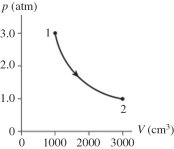
(Multiple Choice)
4.8/5  (37)
(37)
Solar houses use a variety of energy storage devices to retain the heat absorbed during the day so that it can be released during the night. Suppose that you were to use a device of this kind to produce steam at 100°C during the day, and then allow the steam to cool to 0°C and freeze during the night. How many kilograms of water would be needed to store 20.0 kWh of energy in this way? The latent heat of vaporization of water is 22.6 × 105 J/kg, the latent heat of fusion of water is 33.5 × 104 J/kg, and the specific heat capacity of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.9/5  (18)
(18)
The temperature of an ideal gas in a sealed rigid 0.60- container is reduced from 460 K to The final pressure of the gas is The molar heat capacity at constant volume of the gas is 28.0 J/mol ∙ K. How much heat is absorbed by the gas during this process? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (34)
(34)
The density of water at 0°C is 999.84 kg/m3 and at 4°C it is 999.96 kg/m3. A 1.0-L container, full to the brim with water at 4.0°C is placed in the refrigerator. By the time that the temperature of the water reaches 0.0°C, what volume of water has spilled from the container, assuming that the contraction of the container is negligible?
(Multiple Choice)
4.7/5  (40)
(40)
A compression at a constant pressure of 200 kPa is performed on 8.00 moles of an ideal monatomic gas. The compression reduces the volume of the gas from to How much work was done by the gas during this process?
(Multiple Choice)
4.7/5  (40)
(40)
A glass flask has a volume of 500 mL at a temperature of 20° C. The flask contains 492 mL of mercury at an equilibrium temperature of 20°C. The temperature is raised until the mercury reaches the 500 mL reference mark. At what temperature does this occur? The coefficients of volume expansion of mercury and glass are 18 ×10-5 K-1 (mercury) and 2.0 ×10-5 K-1 (glass).
(Multiple Choice)
4.8/5  (32)
(32)
A heat-conducting rod that is wrapped in insulation is constructed with a 0.15-m length of alloy A and a 0.40-m length of alloy B, joined end-to-end. Both pieces have cross-sectional areas of 0.0020 m2. The thermal conductivity of alloy B is known to be 1.8 times as great as that for alloy A. The end of the rod in alloy A is maintained at a temperature of 10°C, and the other end of the rod is maintained at an unknown temperature. When steady state flow has been established, the temperature at the junction of the alloys is measured to be 40° C, and the rate of heat flow in the rod is measured at 56 W. What is the temperature of the end of the rod in alloy B?
(Multiple Choice)
4.9/5  (29)
(29)
An aluminum electric tea kettle with a mass of 500 g is heated with a 500-W heating coil. How long will it take to heat up 1.0 kg of water from 18°C to 98°C in the tea kettle? The specific heat of aluminum is 900 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.9/5  (42)
(42)
A gas expands from an initial volume of 30.0 L to a final volume of 65.0 L at a constant pressure of 110 kPa. How much work is done by the gas during this expansion?
(Multiple Choice)
4.9/5  (34)
(34)
The figure shows a graph of the temperature of a pure substance as a function of time as heat is added to it at a constant rate in a closed container. If LF is the latent heat of fusion of this substance and LV is its latent heat of vaporization, what is the value of the ratio LV/LF? 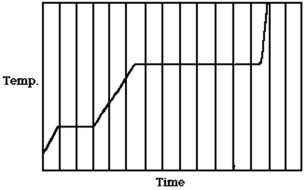
(Multiple Choice)
5.0/5  (37)
(37)
Showing 201 - 220 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)