Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension174 Questions
Exam 3: Vectors and Motion in Two Dimensions183 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity73 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work223 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids115 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics155 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces95 Questions
Exam 21: Electric Potential144 Questions
Exam 22: Current and Resistance125 Questions
Exam 23: Circuits157 Questions
Exam 24: Magnetic Fields and Forces168 Questions
Exam 25: EM Induction and Em Waves185 Questions
Exam 26: AC Electricity122 Questions
Exam 27: Relativity126 Questions
Exam 28: Quantum Physics86 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A piece of iron of mass 0.12 kg is taken from an oven where its temperature is 336°C and quickly placed in an insulated copper can that contains 0.20 kg of water. The copper can has mass 0.50 kg, and it and the water in it are originally at a temperature of 20°C. Calculate the final temperature of the system, assuming no heat is lost to the surroundings. Use the following specific heats: 4190J/kg ∙ C° (water), 470 J/kg ∙ C° (iron), and 390 J/kg ∙ C° (copper).
(Short Answer)
4.7/5  (43)
(43)
An ideal gas occupies 6.00 × 102 cm3 at 20°C. At what temperature will it occupy 1.20 × 103 cm3 if the pressure is held constant?
(Multiple Choice)
4.8/5  (26)
(26)
The molecular weight of nitrogen, N2, is 28 g/mol. What is the rms speed of nitrogen molecules in a cooler at 8.0°C? The Boltzmann constant is 1.38 × 10-23 J/K and NA = 6.022 × 1023 molecules/mol.
(Multiple Choice)
4.9/5  (32)
(32)
A beaker of negligible heat capacity contains 456 g of ice at -25.0°C. A lab technician begins to supply heat to the container at the rate of 1000 J/min. How long after starting will it take before the temperature starts to rise above 0°C? The specific heat of ice is 2090 J/kg ∙ K and the latent heat of fusion of water is 33.5 × 104 J/kg.
(Short Answer)
4.9/5  (42)
(42)
An architect is interested in estimating the rate of heat loss, ΔQ/Δt, through a sheet of insulating material as a function of the thickness of the sheet. Assuming fixed temperatures on the two faces of the sheet and steady state heat flow, which one of the graphs shown in the figure best represents the rate of heat transfer as a function of the thickness of the insulating sheet? 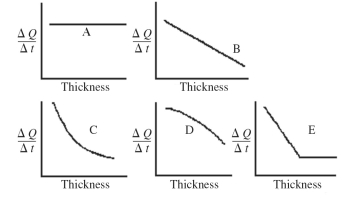
(Multiple Choice)
4.8/5  (28)
(28)
Object 1 has three times the specific heat capacity and four times the mass of Object 2. The two objects are given the same amount of heat. If the temperature of Object 1 changes by an amount ΔT, the change in temperature of Object 2 will be
(Multiple Choice)
4.8/5  (34)
(34)
A jar holds 2.0 L of ideal nitrogen gas, N2, at STP. The atomic mass of nitrogen is 14.0 g/mol, the ideal gas constant is R = 8.31 J/mol ∙ K, Avogadro's number is NA = 6.022 × 1023 molecules/mol, and 1.00 atm = 101 kPa.
(a) How many moles of nitrogen are in the jar?
(b) How many nitrogen molecules are in the jar?
(c) What is the mass of the nitrogen in the jar?
(Short Answer)
4.8/5  (36)
(36)
The root-mean-square speed of the molecules of an ideal gas is v. The gas is now slowly compressed to one-half its original volume with no change in temperature. What is the root-mean-square speed of the molecules now?
(Multiple Choice)
4.8/5  (33)
(33)
How many moles are there in 2.00 kg of copper? The atomic weight of copper is 63.5 g/mol and its density is 8.90 g/cm3.
(Multiple Choice)
4.8/5  (39)
(39)
In a given reversible process, the temperature of an ideal gas is kept constant as the gas is compressed to a smaller volume. Which one of the following statements about the gas is correct?
(Multiple Choice)
4.7/5  (34)
(34)
A steel bridge is 1000 m long at -20°C in winter. What is the change in length when the temperature rises to 40°C in summer? The average coefficient of linear expansion of this steel is 11 × 10-6 K-1.
(Multiple Choice)
4.8/5  (31)
(31)
A 0.40- gas tank holds 7.0 moles of ideal diatomic nitrogen gas at a temperature of The atomic mass of nitrogen is . What is the pressure of the gas? (R = 8.31 J/mol ∙ K, 1 atm = 101 kPa)
(Multiple Choice)
4.9/5  (30)
(30)
A sealed cylinder fitted with a movable piston contains ideal gas at 27°C, pressure 0.500 × 105 Pa, and volume 1.25 m3. What will be the final temperature if the gas is compressed to 0.800 m3 and the pressure rises to 0.820 × 105 Pa?
(Multiple Choice)
4.8/5  (39)
(39)
The figure shows a pV diagram for 2.9 g of ideal oxygen gas O2 in a sealed container. The temperature of state 1 is 76° C, the atomic mass of the oxygen atom is 16 g/mol, and R = 8.31 J/mol ∙ K. What are the temperatures T3 and T4? 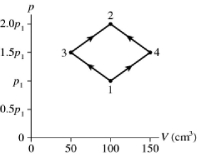
(Multiple Choice)
4.8/5  (33)
(33)
A laboratory vacuum pump can reduce the pressure in a chamber to 1.0 × 10-7 Pa. If the volume of the chamber is 0.500 m3 and the temperature is 27°C, how many molecules are left inside the chamber? (NA = 6.022 × 1023 molecules/mol, R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.9/5  (35)
(35)
A lab assistant drops a 400.0-g piece of metal at 100.0°C into a 100.0-g aluminum cup containing 500.0 g of water at In a few minutes, she measures the final temperature of the system to be 40.0°C. What is the specific heat of the 400.0-g piece of metal, assuming that no significant heat is exchanged with the surroundings? The specific heat of this aluminum is 900.0 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.9/5  (33)
(33)
If 50 g of lead (of specific heat 0.11 kcal/kg ∙ C°) at 100°C is put into 75 g of water (of specific heat 1.0 kcal/kg ∙ C°) at 0°C. What is the final temperature of the mixture?
(Multiple Choice)
4.8/5  (35)
(35)
If you wanted to know how much the temperature of a particular piece of material would rise when a known amount of heat was added to it, which of the following quantities would be most helpful to know?
(Multiple Choice)
4.7/5  (33)
(33)
A glass tea kettle containing 500 g of water is on the stove. The portion of the tea kettle that is in contact with the heating element has an area of 0.090 m2 and is 1.5 mm thick. At a certain moment, the temperature of the water is 75°C, and it is rising at the rate of 3 C° per minute. What is the temperature of the outside surface of the bottom of the tea kettle? Neglect the heat capacity of the kettle, and assume that the inner surface of the kettle is at the same temperature as the water inside. The thermal conductivity of glass is 0.840 W/m ∙ K and the specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.9/5  (26)
(26)
If the temperature of a gas is increased from 20°C to 100°C, by what factor does the rms speed of an ideal molecule change?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 41 - 60 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)