Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
Which of the following has a standard enthalpy of formation value of zero at 25°C?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following thermodynamic quantities are state functions: heat (q),work (w),enthalpy change ( H),and/or internal energy change ( U)?
(Multiple Choice)
4.9/5  (33)
(33)
Which of these physical changes would require the release of energy?
(Multiple Choice)
4.8/5  (39)
(39)
When 0.236 mol of a weak base (A-)is reacted with excess HCl,6.91 kJ of energy is released as heat.What is H for this reaction per mole of A- consumed?
(Multiple Choice)
4.7/5  (38)
(38)
If 50.0 g of benzene,C6H6,at 25.0 C absorbs 2.71 kJ of energy in the form of heat,what is the final temperature of the benzene? The specific heat capacity of benzene is 1.72 J/g.K.
(Multiple Choice)
4.9/5  (27)
(27)
When 50.0 mL of 1.30 M of HCl(aq)is combined with 50.0 mL of 1.20 M of NaOH(aq)in a coffee-cup calorimeter,the temperature of the solution increases by 8.01°C.What is the change in enthalpy for this balanced reaction?
HCl(aq)+ NaOH(aq) NaCl(aq)+ H2O(l)
Assume that the solution density is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g. C.
(Multiple Choice)
4.7/5  (43)
(43)
Using the following thermochemical data:
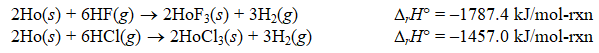 Calculate rH for the following reaction:
HoF3(s)+ 3HCl(g) HoCl3(s)+ 3HF(g)
Calculate rH for the following reaction:
HoF3(s)+ 3HCl(g) HoCl3(s)+ 3HF(g)
(Multiple Choice)
4.9/5  (36)
(36)
Given the thermochemical equation 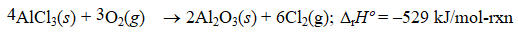 Find rHº for the following reaction.
Find rHº for the following reaction.
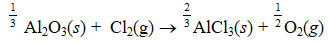
(Multiple Choice)
4.7/5  (40)
(40)
Determine rH for the following reaction,
2 NH3(g)+ 5/2 O2(g) 2 NO(g)+ 3 H2O(g)
Given the thermochemical equations below.
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following processes is/are exothermic?
1)the reaction of butane with oxygen
2)the melting of gold
3)cooling copper from 225 C to 65 C
(Multiple Choice)
4.8/5  (30)
(30)
If q = 98 kJ and w = 4 kJ for a certain process,that process
(Multiple Choice)
4.7/5  (41)
(41)
Dry ice converts directly from a solid to a gas when heated.This process is called ________.
(Short Answer)
4.8/5  (37)
(37)
Showing 61 - 74 of 74
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)