Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions
Exam 1: Basic Concepts of Chemistry53 Questions
Exam 2: Mathlets Review: The Tools of Quantitative Chemistry78 Questions
Exam 3: Atoms, molecules, and Ions105 Questions
Exam 4: Chemical Reactions85 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions76 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions74 Questions
Exam 7: The Structure of Atoms73 Questions
Exam 8: The Structure of Atoms and Periodic Trends82 Questions
Exam 9: Bonding and Molecular Structure97 Questions
Exam 10: Bonding and Molecular Structure: Orbital Hybridization and Molecular Orbitals72 Questions
Exam 11: Gases and Their Properties92 Questions
Exam 12: Intermolecular Forces and Liquids79 Questions
Exam 13: The Chemistry of Solids77 Questions
Exam 14: Solutions and Their Behavior83 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions84 Questions
Exam 16: Principles of Reactivity: Chemical Equilibria82 Questions
Exam 17: The Chemistry of Acids and Bases93 Questions
Exam 18: Principles of Reactivity: Other Aspects of Aqueous Equilibria88 Questions
Exam 19: Entropy and Free Energy76 Questions
Exam 20: Principles of Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry Earths Environment,energy,and Sustainability48 Questions
Exam 22: The Chemistry of the Main Group Elements84 Questions
Exam 23: The Chemistry of the Transition Elements82 Questions
Exam 24: Carbon: Not Just Another Element93 Questions
Exam 25: Biochemistry54 Questions
Exam 26: Nuclear Chemistry79 Questions
Select questions type
A 41.3-g piece of nickel (s = 0.443 J/(g·°C)),initially at 255.8°C,is added to 144.8 g of a liquid,initially at 23.1°C,in an insulated container.The final temperature of the metal-liquid mixture at equilibrium is 35.4°C.What is the identity of the liquid? Neglect the heat capacity of the container.
(Multiple Choice)
4.9/5  (42)
(42)
Hydrazine,N2H4,is a liquid used as a rocket fuel.It reacts with oxygen to yield nitrogen gas and water.
N2H4( 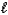 )+ O2(g) N2(g)+ 2 H2O(
)+ O2(g) N2(g)+ 2 H2O( 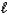 )
The reaction of 6.50 g N2H4 evolves 126.2 kJ of heat.Calculate the enthalpy change per mole of hydrazine combusted.
)
The reaction of 6.50 g N2H4 evolves 126.2 kJ of heat.Calculate the enthalpy change per mole of hydrazine combusted.
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following processes will result in the lowest final temperature of the metal-water mixture at when thermal equilibrium is reached? The specific heat capacity of nickel is 0.443 J/(g·°C).The specific heat capacity of water is 4.184 J/(g·°C).
(Multiple Choice)
4.7/5  (43)
(43)
Internal energy and enthalpy are state functions.What is meant by this statement?
(Essay)
4.8/5  (38)
(38)
Calculate the energy in the form of heat (in kJ)required to change 71.8 g of liquid water at 25.7 C to ice at -16.1 C.Assume that no energy in the form of heat is transferred to the environment.(Heat of fusion = 333 J/g; heat of vaporization = 2256 J/g; specific heat capacities: ice = 2.06 J/g.K,liquid water = 4.184 J/g.K)
(Multiple Choice)
4.8/5  (37)
(37)
Determine the enthalpy change for the decomposition of calcium carbonate
CaCO3(s) CaO(s)+ CO2(g)
Given the thermochemical equations below.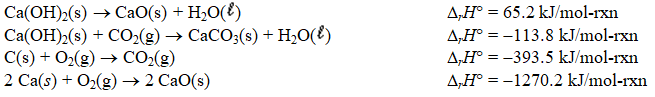
(Multiple Choice)
4.8/5  (41)
(41)
A 170.0-g sample of metal at 79.00°C is added to 170.0 g of H2O(l)at 14.00°C in an insulated container.The temperature rises to 16.19°C.Neglecting the heat capacity of the container,what is the specific heat capacity of the metal? The specific heat capacity of H2O(l)is 4.18 J/(g·°C).
(Multiple Choice)
4.8/5  (38)
(38)
Determine the standard enthalpy of formation of Fe2O3(s)given the thermochemical equations below.
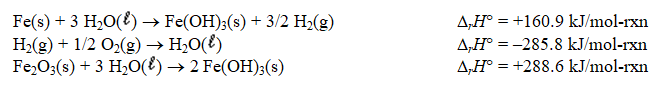
(Multiple Choice)
4.9/5  (43)
(43)
What is the standard enthalpy of formation of BaCO3(s)?
BaO(s)+ CO2(g) BaCO3(s); H° = -269.3 kJ/mol-rxn
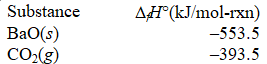
(Multiple Choice)
4.9/5  (32)
(32)
What is the minimum mass of ice at 0.0 C that must be added to 1.00 kg of water to cool the water from 28.0 C to 12.0 C? (Heat of fusion = 333 J/g; specific heat capacities: ice = 2.06 J/g.K,liquid water = 4.184 J/g.K)
(Multiple Choice)
5.0/5  (40)
(40)
Calculate U of a gas for a process in which the gas absorbs 19 J of heat and does 49 J of work by expanding.
(Multiple Choice)
4.8/5  (37)
(37)
A 100 g sample of each of the following metals is heated from 35 C to 45 C.Which metal absorbs the greatest amount of heat energy?

(Multiple Choice)
4.9/5  (35)
(35)
How much energy is needed to convert 57.5 grams of ice at 0.00°C to liquid water at 75.0°C?
Specific heat capacity (ice)= 2.10 J/g°C
Specific heat capacity (liquid water)= 4.18 J/g°C
Heat of fusion = 333 J/g
Heat of vaporization = 2258 J/g
(Multiple Choice)
4.8/5  (43)
(43)
How much energy is gained by nickel when 18.7 g of nickel is warmed from 20.5 °C to 79.8 °C? The specific heat capacity of nickel is 0.443 J/(g·°C).
(Multiple Choice)
4.9/5  (38)
(38)
Acetylene,C2H2,is a gas used in welding.The molar enthalpy of combustion for acetylene is -2599 kJ.A mass of 0.338 g C2H2(g)is combusted in a bomb calorimeter.If the heat capacity of the calorimeter is 729 J/K and it contains 1.150 kg of water,what is the temperature increase of the bomb calorimeter? The specific heat capacity of water is 4.184 J/g.K and the molar mass of acetylene is 26.04 g/mol.
(Multiple Choice)
4.7/5  (37)
(37)
The heat of vaporization of benzene,C6H6,is 30.7 kJ/mol at its boiling point of 80.1 C.How much energy in the form of heat is required to vaporize 102 g benzene at its boiling point?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following statements is/are CORRECT?
1)If a reaction occurs at constant pressure,q = H.
2)The change in energy for a system is defined as the sum of the energies transferred as heat and work (i.e., U = q + w).
3)If a reaction occurs at constant volume,q = w
(Multiple Choice)
4.9/5  (35)
(35)
A hot piece of iron is dropped into a beaker containing colder water.Which of the following statements is/are CORRECT?
1)Energy is transferred as heat from the iron to the water.
2)Thermal equilibrium is attained when the iron and the water reach the same temperature.
3)Thermal energy from the iron is converted to electrostatic energy in the water.
(Multiple Choice)
4.9/5  (34)
(34)
Commercial cold packs consist of solid ammonium nitrate and water.NH4NO3 absorbs 25.69 kJ of heat per mole dissolved in water.In a coffee-cup calorimeter,5.60 g NH4NO3 is dissolved in 100.0 g of water at 22.0 C.What is the final temperature of the solution? Assume that the solution has a specific heat capacity of 4.18 J/g.K.
(Multiple Choice)
4.9/5  (32)
(32)
Showing 41 - 60 of 74
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)