Exam 12: Radicals
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding90 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry43 Questions
Exam 3: An Introduction to Organic Compounds: Nomenclature, physical Properties, and Structure136 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space125 Questions
Exam 5: Alkenes: Structure,nomenclature,and an Introduction to Reactivity - Thermodynamics and Kinetics84 Questions
Exam 6: The Reactions of Alkenes - the Stereochemistry of Addition Reactions89 Questions
Exam 7: The Reactions of Alkynes - Introduction to Multistep Synthesis124 Questions
Exam 8: Delocalized Electrons: Their Effect on Stability, pka, and the Products of a Reaction - Aromaticity and Electronic Effects: an Introduction to the Reactions of Benzene185 Questions
Exam 9: Substitution and Elimination Reactions of Alkyl Halides228 Questions
Exam 10: Reactions of Alcohols, ethers, epoxides, amines and Sulfur-Containing Compounds109 Questions
Exam 11: Organometallic Compounds65 Questions
Exam 12: Radicals141 Questions
Exam 13: Mass Spectrometry,infrared Spectroscopy,and Uvvis Spectroscopy140 Questions
Exam 14: Nmr Spectroscopy122 Questions
Exam 15: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives126 Questions
Exam 16: Reactions of Aldehydes and Ketones122 Questions
Exam 17: Reactions at the Α-Carbon121 Questions
Exam 18: Reactions of Benzene and Substituted Benzenes168 Questions
Exam 19: More About Amines - Reactions of Heterocylic Compounds126 Questions
Exam 20: The Organic Chemistry of Carbohydrates110 Questions
Exam 21: Amino Acids,peptides,and Proteins117 Questions
Exam 22: Catalysis in Organic Reactions and in Enzymatic Reactions92 Questions
Exam 23: The Organic Chemistry of the Coenzymes, compounds Derived From Vitamins102 Questions
Exam 24: The Organic Chemistry of the Metabolic Pathways90 Questions
Exam 25: The Organic Chemistry of Lipids37 Questions
Exam 26: The Chemistry of the Nucleic Acids94 Questions
Exam 27: Synthetic Polymers116 Questions
Exam 28: Pericyclic Reactions102 Questions
Select questions type
How would you convert 1-butene to 1-butanol using radical chemistry?
(Essay)
4.9/5  (36)
(36)
Which of the following is the initiation step for the monobromination of cyclohexane? 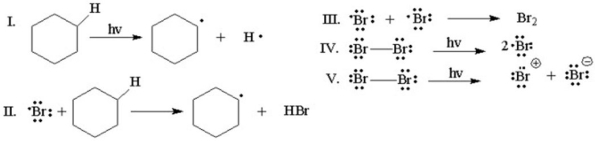
(Multiple Choice)
4.8/5  (30)
(30)
How many distinct monochlorinated products,including stereoisomers,can result when the alkane below is heated in the presence of Cl2? 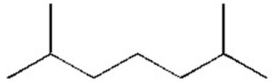
(Multiple Choice)
4.8/5  (37)
(37)
Calculate the overall ΔH° for the reaction shown given the bond dissociation energies (in kcal/mol)below: (CH3)3C-H + Cl-Cl → (CH3)3C-Cl + H-Cl
91 58 78.5 103
(Multiple Choice)
4.9/5  (36)
(36)
When light is shown on a mixture of chlorine and chloromethane,carbon tetrachloride is one of the components of the final reaction mixture.Propose a series of mechanistic steps which explain this observation.
(Essay)
4.9/5  (40)
(40)
Calculate the percentage of 1-chloro-3-methylbutane in the following reaction. 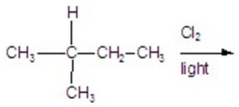
(Multiple Choice)
4.8/5  (37)
(37)
Which H in the molecule below is removed to generate the most stable carbon radical? Show the structure of this radical. 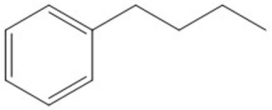
(Essay)
4.9/5  (45)
(45)
A hydrocarbon with molecular formula C5H12 is subjected to free radical chlorination conditions and only one monochlorinated product resulted.Provide the structure of this product.
(Essay)
4.8/5  (38)
(38)
What reagent can best be used to convert cyclopentene to 3-bromocyclopentene in a single step?
(Multiple Choice)
4.7/5  (29)
(29)
When (R)-2-chlorobutane reacts with Br2/hv,which of the following is true? 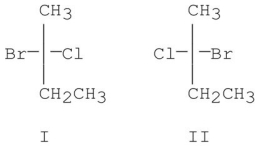
(Multiple Choice)
4.8/5  (26)
(26)
Which of the halogens below undergoes free radical halogenation with ethane most rapidly?
(Multiple Choice)
4.9/5  (33)
(33)
Provide the reagents necessary for carrying out the transformation of cyclopentane to cyclopentene.
(Short Answer)
4.7/5  (37)
(37)
If cyclohexane reacts with excess Cl2 at high temperature,how many distinct dichlorocyclohexane products are possible? Include all stereoisomers.
(Multiple Choice)
4.9/5  (29)
(29)
Calculate the percentage of 2-chloro-2-methyl butane in the following reaction. 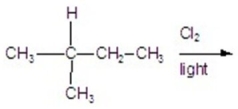
(Multiple Choice)
4.9/5  (36)
(36)
The reaction Br2 + CH3Br → CH2Br2 + HBr was carried out.Which of the following mechanism steps is both productive and relatively likely to occur?
(Multiple Choice)
4.8/5  (29)
(29)
Showing 101 - 120 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)