Exam 12: Radicals
Exam 1: Remembering General Chemistry: Electronic Structure and Bonding90 Questions
Exam 2: Acids and Bases: Central to Understanding Organic Chemistry43 Questions
Exam 3: An Introduction to Organic Compounds: Nomenclature, physical Properties, and Structure136 Questions
Exam 4: Isomers: the Arrangement of Atoms in Space125 Questions
Exam 5: Alkenes: Structure,nomenclature,and an Introduction to Reactivity - Thermodynamics and Kinetics84 Questions
Exam 6: The Reactions of Alkenes - the Stereochemistry of Addition Reactions89 Questions
Exam 7: The Reactions of Alkynes - Introduction to Multistep Synthesis124 Questions
Exam 8: Delocalized Electrons: Their Effect on Stability, pka, and the Products of a Reaction - Aromaticity and Electronic Effects: an Introduction to the Reactions of Benzene185 Questions
Exam 9: Substitution and Elimination Reactions of Alkyl Halides228 Questions
Exam 10: Reactions of Alcohols, ethers, epoxides, amines and Sulfur-Containing Compounds109 Questions
Exam 11: Organometallic Compounds65 Questions
Exam 12: Radicals141 Questions
Exam 13: Mass Spectrometry,infrared Spectroscopy,and Uvvis Spectroscopy140 Questions
Exam 14: Nmr Spectroscopy122 Questions
Exam 15: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives126 Questions
Exam 16: Reactions of Aldehydes and Ketones122 Questions
Exam 17: Reactions at the Α-Carbon121 Questions
Exam 18: Reactions of Benzene and Substituted Benzenes168 Questions
Exam 19: More About Amines - Reactions of Heterocylic Compounds126 Questions
Exam 20: The Organic Chemistry of Carbohydrates110 Questions
Exam 21: Amino Acids,peptides,and Proteins117 Questions
Exam 22: Catalysis in Organic Reactions and in Enzymatic Reactions92 Questions
Exam 23: The Organic Chemistry of the Coenzymes, compounds Derived From Vitamins102 Questions
Exam 24: The Organic Chemistry of the Metabolic Pathways90 Questions
Exam 25: The Organic Chemistry of Lipids37 Questions
Exam 26: The Chemistry of the Nucleic Acids94 Questions
Exam 27: Synthetic Polymers116 Questions
Exam 28: Pericyclic Reactions102 Questions
Select questions type
Consider the reaction: CH3CH2 ∙ + Br2 → CH3CH2Br + Br ∙.Given that this reaction has an activation energy of +6 kcal/mol and a ΔH° of -22 kcal/mol,sketch a reaction-energy profile for this reaction.Label the axes and show Ea and ΔH° on your drawing.
(Essay)
4.8/5  (31)
(31)
The reaction Br2 + CH3Br → CH2Br2 + HBr was carried out.Which of the following mechanism steps is productive,but relatively unlikely to occur?
(Multiple Choice)
4.7/5  (34)
(34)
A sample of (R)-2-chlorobutane, 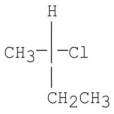 reacts with Br2 in the presence of light,and all the products having the formula C4H8BrCl were isolated.Two possible isomers are shown below:
reacts with Br2 in the presence of light,and all the products having the formula C4H8BrCl were isolated.Two possible isomers are shown below:  Of these
Of these
(Multiple Choice)
4.8/5  (39)
(39)
When butane undergoes free radical bromination,the product mixture contains 98% 2-bromobutane and 2% 1-bromobutane.How many times more susceptible to hydrogen atom abstraction is a secondary hydrogen in butane than is a primary hydrogen?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following does not occur in a propagation step in the free radical bromination of ethane to form bromoethane?
(Multiple Choice)
4.9/5  (26)
(26)
Which of the following reactions is a termination step in the free radical chlorination of methane?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following products result from the disproportionation reaction between two propyl radicals? 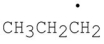
(Multiple Choice)
4.7/5  (29)
(29)
Which of the following represents the best preparation of 2-cyclopentenol from cyclopentane?
(Multiple Choice)
4.8/5  (38)
(38)
Consider the following monobromination reaction,then answer the following questions. 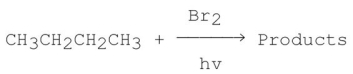 a)Give the structures and the IUPAC names for the products.
b)Give the common names for the products.
c)Calculate ΔH° for the overall reaction using the following data for the indicated bond dissociation energies:
a)Give the structures and the IUPAC names for the products.
b)Give the common names for the products.
c)Calculate ΔH° for the overall reaction using the following data for the indicated bond dissociation energies:  d)Calculate the percent yield for each product.(relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
e)Propose a step-by-step mechanism for the major product only.
f)Draw a schematic potential energy diagram for the rate-determining step (RDS)only.
g)Does the transition state of the RDS resemble more closely the reactants or the products?
h)Would the value of the activation energy be different for different alkanes? Explain.
i)Would the reaction slow down or speed up if I2 is used instead of Br2? Explain.
d)Calculate the percent yield for each product.(relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
e)Propose a step-by-step mechanism for the major product only.
f)Draw a schematic potential energy diagram for the rate-determining step (RDS)only.
g)Does the transition state of the RDS resemble more closely the reactants or the products?
h)Would the value of the activation energy be different for different alkanes? Explain.
i)Would the reaction slow down or speed up if I2 is used instead of Br2? Explain.
(Essay)
4.7/5  (24)
(24)
What sequence of reagents can be used to convert 3,3-dimethylpentane into 3,3-dimethyl-2-pentanone?
(Essay)
4.8/5  (38)
(38)
An unknown sample is suspected of being either ethane or isobutane.How would you distinguish between the two alkanes?
(Essay)
4.9/5  (33)
(33)
Provide the structure of the major organic product of the following reaction. 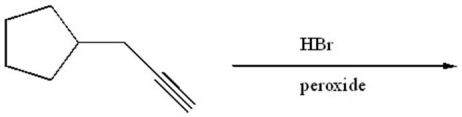
(Essay)
4.8/5  (40)
(40)
Showing 41 - 60 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)