Exam 23: Nuclear Reactions and Their Applications
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Select the nuclide that completes the following nuclear reaction. 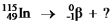
Free
(Multiple Choice)
4.7/5  (37)
(37)
Correct Answer:
B
Bombardment of uranium-238 nuclei by carbon-12 nuclei produces californium-246 and neutrons.Write a complete,balanced equation for this nuclear process.
Free
(Essay)
4.8/5  (36)
(36)
Correct Answer:
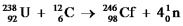
Select the nuclide that completes the following nuclear reaction. 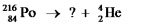
(Multiple Choice)
4.8/5  (35)
(35)
Calcium-39 undergoes positron decay.Each positron carries 5.49 MeV of energy.How much energy will be emitted when 0.0025 mol of calcium-39 decays?
(Multiple Choice)
4.8/5  (31)
(31)
A 7.85 10-5 mol sample of copper-61 emits 1.47 1019 positrons in 90.0 minutes.What is the decay constant for copper-61?
(Multiple Choice)
4.8/5  (27)
(27)
Fill in missing sub- and superscripts for all particles to complete the following equation for alpha decay. 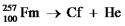
(Essay)
4.8/5  (41)
(41)
Identify the missing species in the following nuclear transmutation. 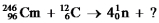
(Multiple Choice)
4.8/5  (42)
(42)
Select the nuclide that completes the following nuclear reaction. 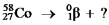
(Multiple Choice)
4.8/5  (38)
(38)
The isotopes 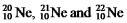 Are all stable,while
Are all stable,while 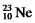 Is radioactive.The mode of decay for
Is radioactive.The mode of decay for 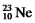 Is most likely to be
Is most likely to be
(Multiple Choice)
4.8/5  (34)
(34)
Select the nuclide that completes the following nuclear reaction. 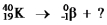
(Multiple Choice)
4.8/5  (32)
(32)
The masses of a potassium-40 atom,a proton and a neutron are 39.963999 amu,1.007825 amu and 1.008665 amu,respectively.Calculate to four significant figures
a.the mass defect in amu and
b.the energy released in MeV/nucleon,in the formation of 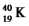 from the appropriate number of protons and neutrons.
from the appropriate number of protons and neutrons.
(Short Answer)
4.8/5  (37)
(37)
The isotope 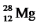 Has a half-life of 21 hours.If a sample initially contains exactly 10000 atoms of
Has a half-life of 21 hours.If a sample initially contains exactly 10000 atoms of 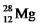 ,approximately how many of these atoms will remain after one week?
,approximately how many of these atoms will remain after one week?
(Multiple Choice)
4.9/5  (35)
(35)
Which one of the following is an incorrect representation of the indicated particle or nucleus?
(Multiple Choice)
4.7/5  (27)
(27)
Identify the missing species in the following nuclear transmutation. 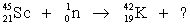
(Multiple Choice)
4.9/5  (36)
(36)
A 9.52 10-5 mol sample of rubidium-86 emits 8.87 1016 particles in one hour.What is the half-life of rubidium-86?
(Multiple Choice)
4.8/5  (38)
(38)
Explain how the number of protons and neutrons in a radioactive nucleus can be used to predict its probable mode of decay.Illustrate your answer with a schematic graph,properly labeled,showing stable nuclides (nuclei)in relation to number of protons and neutrons.
(Essay)
4.9/5  (35)
(35)
Showing 1 - 20 of 75
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)