Exam 6: Thermochemistry: Energy Flow and Chemical Change
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
The standard state of a substance in aqueous solution is a 1 M solution.
Free
(True/False)
4.8/5  (31)
(31)
Correct Answer:
True
A system which undergoes an adiabatic change and has work done on it by the surroundings has
Free
(Multiple Choice)
4.7/5  (30)
(30)
Correct Answer:
A
A system receives 575 J of heat and delivers 425 J of work.Calculate the change in the internal energy, E,of the system.
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
B
Consider the equation E = q + w
Explain fully the meaning of all three terms in the equation,and also the implied sign convention for q and w.
(Essay)
4.8/5  (30)
(30)
Nitric acid,which is among the top 15 chemicals produced in the United States,was first prepared over 1200 years ago by heating naturally occurring sodium nitrate (called saltpeter)with sulfuric acid and collecting the vapors produced.Calculate H°rxn for this reaction. H°f [NaNO3(s)] = -467.8 kJ/mol; H°f [NaHSO4(s)] = -1125.5 kJ/mol; H°f [H2SO4(l)= -814.0 kJ/mol; H°f [HNO3(g)] = -135.1 kJ/mol
NaNO3(s)+ H2SO4(l) NaHSO4(s)+ HNO3(g)
(Multiple Choice)
4.9/5  (27)
(27)
Sand is converted to pure silicon in a three step process.The third step is
SiCl4(g)+ 2Mg(s) 2MgCl2(s)+ Si(s) H = -625.6 kJ
What is the enthalpy change when 25.0 mol of silicon tetrachloride is converted to elemental silicon?
(Multiple Choice)
4.9/5  (29)
(29)
A system that does no work but which transfers heat to the surroundings has
(Multiple Choice)
4.8/5  (37)
(37)
A common laboratory reaction is the neutralization of an acid with a base.When 50.0 mL of 0.500 M HCl at 25.0°C is added to 50.0 mL of 0.500 M NaOH at 25.0°C in a coffee cup calorimeter,the temperature of the mixture rises to 28.2°C.What is the heat of reaction per mole of acid? Assume the mixture has a specific heat capacity of 4.18 J/(g·K)and that the densities of the reactant solutions are both 1.00 g/mL.
(Multiple Choice)
4.7/5  (42)
(42)
Natural gas,or methane,is an important fuel.Combustion of one mole of methane releases 802.3 kilojoules of energy.How much energy does that represent in kilocalories?
(Multiple Choice)
4.9/5  (40)
(40)
A system contracts from an initial volume of 15.0 L to a final volume of 10.0 L under a constant external pressure of 0.800 atm.The value of w,in J,is
(Multiple Choice)
4.8/5  (31)
(31)
Benzene is a starting material in the synthesis of nylon fibers and polystyrene (styrofoam).Its specific heat capacity is 1.74 J/(g·K).If 16.7 kJ of energy is absorbed by a 225-g sample of benzene at 20.0°C,what is its final temperature?
(Multiple Choice)
4.8/5  (23)
(23)
A system delivers 225 J of heat to the surroundings while delivering 645 J of work.Calculate the change in the internal energy, E,of the system.
(Multiple Choice)
4.7/5  (36)
(36)
A Snickers® candy bar contains 280 Calories,of which the fat content accounts for 120 Calories.What is the energy of the fat content,in kJ?
(Multiple Choice)
4.8/5  (33)
(33)
The Starship Enterprise is caught in a time warp and Spock is forced to use the primitive techniques of the 20th century to determine the specific heat capacity of an unknown mineral.The 307-g sample was heated to 98.7°C and placed into a calorimeter containing 72.4 g of water at 23.6°C.The heat capacity of the calorimeter was 15.7 J/K.The final temperature in the calorimeter was 32.4°C.What is the specific heat capacity of the mineral?
(Multiple Choice)
4.9/5  (43)
(43)
Which one of the following statements about standard states is incorrect?
(Multiple Choice)
4.9/5  (28)
(28)
Two solutions (the system),each of 25.0 mL volume and at 25.0°C,are mixed in a beaker.A reaction occurs between them,and the temperature rises to 35.0°C.After the products have equilibrated with the surroundings,the temperature is again 25.0°C and the total volume is 50.0 mL.No gases are involved in the reaction.Which one of the following relationships concerning the change from initial to final states (both at 25.0°C)is correct?
(Multiple Choice)
4.9/5  (30)
(30)
Diborane (B2H6)has been considered as a possible rocket fuel.Calculate H° for the reaction
B2H6(g) 2B(s)+ 3H2(g)
using the following data:
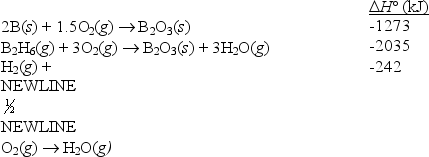
(Short Answer)
4.9/5  (30)
(30)
A mass of 1.250 g of benzoic acid (C7H6O2)was completely combusted in a bomb calorimeter.If the heat capacity of the calorimeter was 10.134 kJ/K and the heat of combustion of benzoic acid is -3226 kJ/mol,calculate (to three decimal places)the temperature increase that should have occurred in the apparatus.
(Essay)
4.8/5  (26)
(26)
a.Starting from the equation H = E + PV,show how the relationship H = qp is derived.Clearly indicate any necessary assumptions or conditions.
b.In one sentence,state in full what is meant by the equation: H = qp.
(Essay)
4.8/5  (34)
(34)
A system expands from a volume of 1.00 L to 2.00 L against a constant external pressure of 1.00 atm.
The work (w)done by the system,in J,is
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 71
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)