Exam 13: The Properties of Solutions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
The mole fraction of potassium nitrate in an aqueous solution is 0.0194.The solution's density is 1.0627 g/mL.Calculate the molarity of the solution.
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
C
Select the weakest electrolyte from the following set.
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
C
The solubility of the oxidizing agent potassium permanganate is 7.1 g per 100.0 g of water at 25°C.What is the mole fraction of potassium permanganate in this solution?
Free
(Multiple Choice)
4.9/5  (30)
(30)
Correct Answer:
A
From the following list of aqueous solutions and water,select the one with the lowest freezing point.
(Multiple Choice)
4.7/5  (38)
(38)
The density of pure water at 25°C is 0.997 g/mL.Considering water as being both solvent and solute,calculate its molarity.
(Short Answer)
4.9/5  (43)
(43)
The heat of solution is the total enthalpy change when a solution is formed from the separated solute and solvent.
(True/False)
4.7/5  (33)
(33)
Methane has a Henry's Law constant (k)of 9.88 10-2 mol/(L·atm)when dissolved in benzene at 25°C.How many grams of CH4 will dissolve in 3.00 L of benzene if the partial pressure of CH4 is 1.48 atm?
(Multiple Choice)
4.9/5  (29)
(29)
Saccharin,one of the first non-nutritive sweeteners used in soft-drinks,is 500 times sweeter than sugar in dilute aqueous solutions.The solubility of saccharin is 1.00 gram per 290 mL of solution.What is the molarity of a saturated saccharin solution? 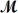 saccharin= 183.2 g/mol
saccharin= 183.2 g/mol
(Multiple Choice)
4.9/5  (46)
(46)
The solubility of gases in water increases with increase in the pressure of the gas.
(True/False)
4.9/5  (33)
(33)
For a given solution,which of the following concentration values will change as temperature changes?
(Multiple Choice)
4.9/5  (33)
(33)
The chemist,Anna Lytic,must prepare 1.00 kg of 15.0% (w/w)acetic acid using a stock solution which is 36.0% (w/w)acetic acid (d = 1.045 g/mL).Which of the following combinations will give her the solution she wants?
(Multiple Choice)
4.9/5  (42)
(42)
Calculate the freezing point of a solution made by dissolving 3.50 g of potassium chloride ( 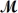 = 74.55 g/mol)in 100.0 g of water.Assume ideal behavior for the solution;Kf = 1.86°C/m.
= 74.55 g/mol)in 100.0 g of water.Assume ideal behavior for the solution;Kf = 1.86°C/m.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following aqueous solutions should demonstrate the most ideal behavior?
(Multiple Choice)
4.8/5  (37)
(37)
Sodium hydroxide is a common ingredient in drain cleaners such as Drano.The mole fraction of sodium hydroxide in a saturated aqueous solution is 0.310.What is the molality of the solution?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following aqueous liquids will have the lowest freezing point?
(Multiple Choice)
4.8/5  (44)
(44)
Which of the following statements describes the correct method of preparation of 1.00 L of a 2.0 M urea solution? 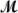 urea = 60.06 g/mol
urea = 60.06 g/mol
(Multiple Choice)
4.9/5  (32)
(32)
Raoult's Law relates the vapor pressure of the solvent above the solution to its mole fraction in the solution.Which of the following is an accurate statement?
(Multiple Choice)
4.8/5  (34)
(34)
A 50.0 % (w/w)solution of sulfuric acid (H2SO4)in water has a density of 1.395 g/mL.What is its molarity?
(Short Answer)
4.9/5  (37)
(37)
A 0.89% (w/v)sodium chloride solution is referred to as physiological saline solution because it has the same concentration of salts as human blood.What is the molarity of a physiological saline solution?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)