Exam 9: Models of Chemical Bonding
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Using appropriate,real examples to illustrate your answer,describe the correlation between bond energy and bond length for a series of varying bond order.
Free
(Essay)
4.8/5  (28)
(28)
Correct Answer:
Carbon and oxygen form single,double and triple bonds.The C=O bond in carbon monoxide is roughly three times as strong as the C-O single bond,while the C=O bond is about twice as strong as the single bond.The bond energy is approximately proportional to the bond order..
Which of the following elements is the least electronegative?
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
E
When one mole of each of the following liquids is burned,which will produce the most heat energy?
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
Describe in brief how electronegativity values can be used to predict the percent ionic character of a bond between two atoms.
(Essay)
4.9/5  (40)
(40)
In not more than three sentences,describe the electron arrangement responsible for bonding in Cl2 molecules.
(Essay)
4.7/5  (26)
(26)
The lattice energy of large ions is greater in magnitude than that of small ions of the same charge.
(True/False)
4.8/5  (34)
(34)
Arrange calcium,rubidium,sulfur,and arsenic in order of decreasing electronegativity.
(Multiple Choice)
4.8/5  (33)
(33)
The lattice energy of CaF2 is the energy change for which one,if any,of the following processes?
(Multiple Choice)
4.9/5  (31)
(31)
When an atom is represented in a Lewis electron dot symbol,the element symbol represents ______________ and the dots represent ______________.
(Short Answer)
4.9/5  (35)
(35)
The electrostatic energy of two charged particles is inversely proportional to the distance between them.
(True/False)
4.8/5  (31)
(31)
Bond energy increases as bond order increases,for bonding between a given pair of atoms.
(True/False)
4.7/5  (35)
(35)
Select the correct formula for a compound formed from barium and nitrogen.
(Multiple Choice)
4.8/5  (33)
(33)
Oxygen difluoride is an unstable molecule that reacts readily with water.Calculate the bond energy of the O-F bond using the standard enthalpy of reaction and the bond energy data provided.
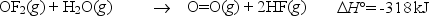

(Short Answer)
4.9/5  (33)
(33)
Arrange aluminum,nitrogen,phosphorus and indium in order of increasing electronegativity.
(Multiple Choice)
4.9/5  (30)
(30)
In which of these substances are the atoms held together by metallic bonding?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following elements is the most electronegative?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following elements is the most electronegative?
(Multiple Choice)
4.8/5  (17)
(17)
Nitrogen and hydrogen combine to form ammonia in the Haber process.Calculate (in kJ)the standard enthalpy change H° for the reaction written below,using the bond energies given.
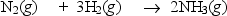 Bond:
N N
H-H
N-H
Bond:
N N
H-H
N-H

(Multiple Choice)
4.9/5  (38)
(38)
Showing 1 - 20 of 60
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)