Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Calculate S° for the reaction
SiCl4(g)+ 2Mg(s) 2MgCl2(s)+ Si(s)
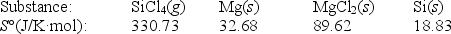
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
B
The temperature at which the following process reaches equilibrium at 1.0 atm is the normal boiling point of hydrogen peroxide.
H2O2(l)  H2O2(g)
Use the following thermodynamic information at 298 K to determine this temperature.
H2O2(g)
Use the following thermodynamic information at 298 K to determine this temperature.
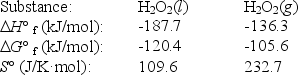
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
B
Which of the following pairs has the member with the greater molar entropy listed first? All systems are at 25°C.
Free
(Multiple Choice)
4.9/5  (32)
(32)
Correct Answer:
C
The water-gas shift reaction plays an important role in the production of clean fuel from coal.
CO(g)+ H2O(g)  CO2(g)+ H2(g)
Use the following thermodynamic data to determine the equilibrium constant Kp at 700.K.
CO2(g)+ H2(g)
Use the following thermodynamic data to determine the equilibrium constant Kp at 700.K.
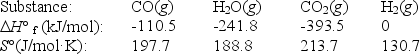
(Short Answer)
4.7/5  (31)
(31)
Which relationship or statement best describes S° for the following reaction?
2H2S(g)+ 3O2(g) 2H2O(g)+ 2SO2(g)
(Multiple Choice)
4.8/5  (36)
(36)
Which,if any,of the following processes is spontaneous under the specified conditions?
(Multiple Choice)
4.8/5  (30)
(30)
In tables of thermodynamic data provided in chemistry books,one finds H° f , G° f and S° listed.Briefly,explain why the entropy data are supplied as S°,while the enthalpy and free energy data are in the form of H° f and G° f,respectively.
(Essay)
4.9/5  (34)
(34)
Sulfuryl dichloride is formed when sulfur dioxide reacts with chlorine.The data refer to 298 K.
SO2(g)+ Cl2(g) SO2Cl2(g)
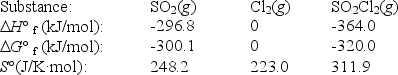 What is the value of G° for this reaction at 600 K?
What is the value of G° for this reaction at 600 K?
(Multiple Choice)
4.8/5  (27)
(27)
Which relationship or statement best describes S° for the following reaction?
Pb(s)+ Cl2(g) PbCl2(s)
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following is necessary for a process to be spontaneous?
(Multiple Choice)
4.7/5  (31)
(31)
Consider the figure below which shows G° for a chemical process plotted against absolute temperature.Which one of the following is an incorrect conclusion,based on the information in the diagram? 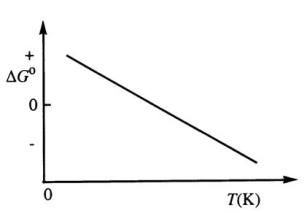
(Multiple Choice)
5.0/5  (34)
(34)
Which relationship or statement best describes S° for the following reaction?
HgS(s)+ O2(g) Hg(l)+ SO2(g)
(Multiple Choice)
4.7/5  (32)
(32)
Elemental boron can be formed by reaction of boron trichloride with hydrogen.
BCl3(g)+ 1.5H2(g) B(s)+ 3HCl(g)
Calculate G° for the reaction.
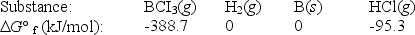
(Multiple Choice)
4.9/5  (31)
(31)
Photosynthesis can be represented by the equation
6CO2(g)+ 6H2O(l) C6H12O6(s)+ 6O2(g)
a.Calculate S° for this process,given the following data:
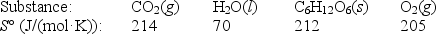 b.Given that H° for the reaction is 2802 kJ,calculate G° at 25°C.
b.Given that H° for the reaction is 2802 kJ,calculate G° at 25°C.
(Essay)
4.9/5  (32)
(32)
Consider the figure below which shows G° for a chemical process plotted against absolute temperature.From this plot,it is reasonable to conclude that: 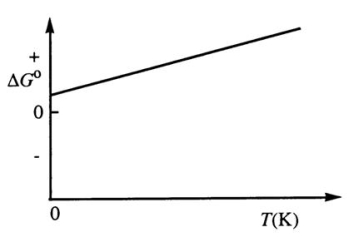
(Multiple Choice)
4.8/5  (36)
(36)
A certain process has Suniv > 0 at 25°C.What does one know about the process?
(Multiple Choice)
4.8/5  (37)
(37)
Which relationship or statement best describes S° for the following reaction?
K2SO4(s) 2K+(aq)+ SO42-(aq)
(Multiple Choice)
4.7/5  (27)
(27)
Showing 1 - 20 of 84
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)