Exam 17: Equilibrium: the Extent of Chemical Reactions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Once a reaction system reaches equilibrium,the concentrations of reactions and products no longer change.
Free
(True/False)
4.9/5  (33)
(33)
Correct Answer:
True
Consider the following gas-phase equilibrium reaction:
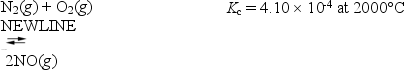 If 1.0 mol of NO is introduced into a 1.0 L container at 2000°C,what is the concentration of NO when equilibrium is reached?
If 1.0 mol of NO is introduced into a 1.0 L container at 2000°C,what is the concentration of NO when equilibrium is reached?
Free
(Essay)
4.8/5  (38)
(38)
Correct Answer:
[NO] = 1.0 10-2 mol L-1
The reaction quotient for a gas phase reaction has a value of 2000.If the number of moles of reactants in the reaction equation is equal to that of the products,which of the following statements is true?
Free
(Multiple Choice)
4.9/5  (45)
(45)
Correct Answer:
D
A mixture 0.500 mole of carbon monoxide and 0.400 mole of bromine was placed into a rigid 1.00-L container and the system was allowed to come to equilibrium.The equilibrium concentration of COBr2 was 0.233 M.What is the value of Kc for this reaction?
CO(g)+ Br2(g)  COBr2(g)
COBr2(g)
(Multiple Choice)
4.8/5  (31)
(31)
Consider the equilibrium:
 Predict and explain how or whether the following actions would affect this equilibrium.
a.adding more solid A
b.lowering the temperature
c.increasing the pressure on the system by reducing its volume
d.adding helium gas to increase the total pressure
Predict and explain how or whether the following actions would affect this equilibrium.
a.adding more solid A
b.lowering the temperature
c.increasing the pressure on the system by reducing its volume
d.adding helium gas to increase the total pressure
(Essay)
4.8/5  (45)
(45)
What is the mass-action expression,Qc,for the following chemical reaction?
Cu2+(aq)+ 4NH3(aq)  Cu(NH3)42+(aq)
Cu(NH3)42+(aq)
(Multiple Choice)
4.8/5  (33)
(33)
The equilibrium constant,Kc ,for the decomposition of COBr2
COBr2(g)  CO(g)+ Br2(g)
Is 0.190.What is Kc for the following reaction?
2CO(g)+ 2Br2(g)
CO(g)+ Br2(g)
Is 0.190.What is Kc for the following reaction?
2CO(g)+ 2Br2(g)  2COBr2(g)
2COBr2(g)
(Multiple Choice)
4.9/5  (37)
(37)
Magnesium hydroxide is used in several antacid formulations.When it is added to water it dissociates into magnesium and hydroxide ions.
Mg(OH)2(s)  Mg2+(aq)+ 2OH-(aq)
The equilibrium constant at 25°C is 8.9 10-12.One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established.What happens to the solution if another 10 grams of Mg(OH)2 are now added to the mixture?
Mg2+(aq)+ 2OH-(aq)
The equilibrium constant at 25°C is 8.9 10-12.One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established.What happens to the solution if another 10 grams of Mg(OH)2 are now added to the mixture?
(Multiple Choice)
4.8/5  (31)
(31)
For a gas-phase equilibrium,a change in the pressure of any single reactant or product will affect the amounts of other substances involved in the equilibrium.
(True/False)
4.7/5  (27)
(27)
At a certain temperature the reaction
CO2(g)+ H2(g)  CO(g)+ H2O(g)
Has Kc = 2.50.If 2.00 mol of carbon dioxide and 1.5 mol of hydrogen are placed in a 5.00 L vessel and equilibrium is established,what will be the concentration of carbon monoxide?
CO(g)+ H2O(g)
Has Kc = 2.50.If 2.00 mol of carbon dioxide and 1.5 mol of hydrogen are placed in a 5.00 L vessel and equilibrium is established,what will be the concentration of carbon monoxide?
(Multiple Choice)
4.9/5  (35)
(35)
The reaction quotient,Qc,for a reaction has a value of 75 while the equilibrium constant,Kc,has a value of 195.Which of the following statements is accurate?
(Multiple Choice)
4.7/5  (34)
(34)
For a gas-phase equilibrium,a change in the pressure of any single reactant or product will change Kp.
(True/False)
4.9/5  (37)
(37)
Write the mass-action expression,Qc,for the following chemical reaction.
NO(g)+ 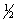 Br2(g)
Br2(g)  NOBr(g)
NOBr(g)
(Multiple Choice)
4.7/5  (37)
(37)
The reaction system
CS2(g)+ 4H2(g)  CH4(g)+ 2H2S(g)
Is at equilibrium.Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
CH4(g)+ 2H2S(g)
Is at equilibrium.Which of the following statements describes the behavior of the system if the partial pressure of hydrogen is doubled?
(Multiple Choice)
4.9/5  (31)
(31)
The following reaction is at equilibrium in a closed container.
CuSO4.5H2O(s)  CuSO4(s)+ 5H2O(g)
Which,if any,of the following actions will lead to an increase in the pressure of H2O present at equilibrium?
CuSO4(s)+ 5H2O(g)
Which,if any,of the following actions will lead to an increase in the pressure of H2O present at equilibrium?
(Multiple Choice)
5.0/5  (33)
(33)
The reaction system
POCl3(g)  POCl(g)+ Cl2(g)
Is at equilibrium.Which of the following statements describes the behavior of the system if the partial pressure of chlorine is reduced by 50%?
POCl(g)+ Cl2(g)
Is at equilibrium.Which of the following statements describes the behavior of the system if the partial pressure of chlorine is reduced by 50%?
(Multiple Choice)
4.8/5  (29)
(29)
The equilibrium constant for the reaction of bromine with chlorine to form bromine monochloride is 58.0 at a certain temperature.
Br2(g)+ Cl2(g)  2BrCl(g)
What is the equilibrium constant for the following reaction?
BrCl(g)
2BrCl(g)
What is the equilibrium constant for the following reaction?
BrCl(g) 
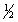 Br2(g)+
Br2(g)+ 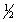 Cl2(g)
Cl2(g)
(Multiple Choice)
4.8/5  (37)
(37)
Consider the equilibrium reaction: H2(g)+ Br2(g)  2HBr(g)
Which of the following correctly describes the relationship between Kc and Kp for the reaction?
2HBr(g)
Which of the following correctly describes the relationship between Kc and Kp for the reaction?
(Multiple Choice)
4.8/5  (37)
(37)
If Q > K,more products need to be formed as the reaction proceeds to equilibrium.
(True/False)
4.7/5  (40)
(40)
Showing 1 - 20 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)