Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
The density of solid sodium chloride,NaCl,is 2.17 g/cm3.Use your knowledge of the sodium chloride lattice to calculate the spacing between Na+ and Cl- nearest neighbors,in cm.(Atomic masses (amu)are: Na,22.99;Cl,35.45.Also,1 amu = 1.661 10-24 g)
Free
(Essay)
4.8/5  (40)
(40)
Correct Answer:
2.82 10-8 cm
Which of the following statements concerning a face-centered cubic unit cell and the corresponding lattice,made up of identical atoms,is incorrect?
Free
(Multiple Choice)
4.7/5  (40)
(40)
Correct Answer:
A
Which of the following statements about the packing of monatomic solids with different unit cells is incorrect?
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
E
A metal such as chromium in the body-centered cubic lattice will have _______________ atom(s)per unit cell.
(Multiple Choice)
4.8/5  (32)
(32)
A temperature increase causes __________________ in the conductivity of a semiconductor.
(Multiple Choice)
4.8/5  (33)
(33)
The energy of a hydrogen bond is greater than that of a typical covalent bond.
(True/False)
5.0/5  (36)
(36)
Select the pair of substances in which the one with the higher vapor pressure at a given temperature is listed first.
(Multiple Choice)
4.9/5  (34)
(34)
Comparing the energies of the following intermolecular forces on a kJ/mol basis,which would normally have the highest energy (i.e. ,be the strongest force)?
(Multiple Choice)
5.0/5  (35)
(35)
Which of the following liquids is likely to have the highest surface tension?
(Multiple Choice)
4.8/5  (31)
(31)
Consider the following phase diagram and identify the process occurring as one goes from point C to point D. 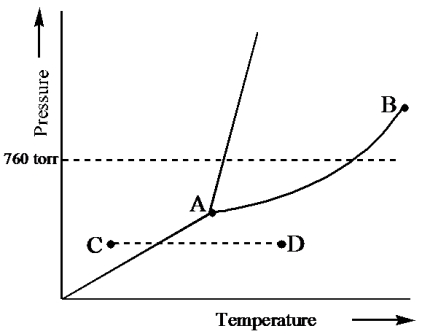
(Multiple Choice)
4.7/5  (40)
(40)
For the solid forms of the following elements,which one is most likely to be of the molecular type?
(Multiple Choice)
4.9/5  (34)
(34)
Lead crystallizes in the face-centered cubic lattice.What is the coordination number for Pb?
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following should have the highest boiling point?
(Multiple Choice)
4.9/5  (33)
(33)
Hexagonal close packing of identical atoms occurs when close-packed layers are stacked in an abcabc....arrangement.
(True/False)
4.8/5  (40)
(40)
Which of the following atoms should have the greatest polarizability?
(Multiple Choice)
4.6/5  (26)
(26)
The phase diagram for xenon has a solid-liquid curve with a positive slope.Which of the following is true?
(Multiple Choice)
4.8/5  (43)
(43)
A cubic unit cell has an edge length of 400.pm.The length of its body diagonal (internal diagonal)in pm is therefore
(Multiple Choice)
4.9/5  (31)
(31)
Assuming that atoms are spherical,calculate the fraction of space which is occupied by atoms (i.e. ,the packing efficiency)in a metal with a simple cubic unit cell.
(Short Answer)
4.7/5  (32)
(32)
In metals,the conduction bands and valence bands of the molecular orbitals are separated by a large energy gap.
(True/False)
4.8/5  (35)
(35)
Showing 1 - 20 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)