Exam 22: The Transition Elements and Their Coordination Compounds
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Apply the valence bond theory to predict the electronic structure and hybridization pattern of chromium in the complex ion Cr(NH3)63+.
Free
(Essay)
4.8/5  (34)
(34)
Correct Answer:
The three d electrons of the Cr(III)will occupy three 3d orbitals,singly.The remaining two 3d orbitals will be hybridized with the 4s and 4p orbitals,yielding six d2sp3 hybrid orbitals,which are used to accommodate the six lone pairs of the ammonia ligands.
In the spectrochemical series,which one of the following ligands has the strongest field?
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
B
The ground state electron configuration of Cr2+ is
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
C
Consider the following octahedral complex structures,each involving ethylene diamine and two different,unidentate ligands X and Y. 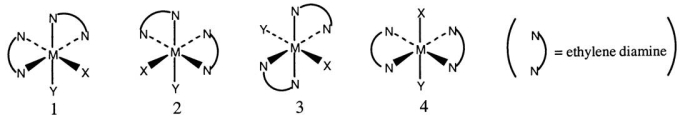 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
(Multiple Choice)
4.8/5  (28)
(28)
Of the 3d transition series of elements,scandium has the greatest atomic radius.
(True/False)
4.8/5  (40)
(40)
If M represents a transition element,which of the following oxides should be the least basic?
(Multiple Choice)
4.7/5  (32)
(32)
Tetrahedral complexes can exhibit both optical and linkage isomerism.
(True/False)
4.8/5  (27)
(27)
In the compound [Ni(en)2(H2O)2]SO4 (where en = ethylenediamine)the oxidation number and coordination number of nickel are,respectively:
(Multiple Choice)
4.9/5  (29)
(29)
The compound Rh(CO)(H)(PH3)2 forms cis and trans isomers.Use this information to predict the geometry of this complex,and draw the geometric isomers.
(Essay)
4.9/5  (40)
(40)
Which of the following transition elements will form an ion with the largest oxidation number?
(Multiple Choice)
4.9/5  (25)
(25)
In a coordination compound involving a complex ion of square planar geometry,which of the following types of isomerism is/are never possible?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following ions could exist in only the high-spin state in an octahedral complex?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following will be the strongest oxidizing agent?
(Multiple Choice)
4.9/5  (36)
(36)
What is the coordination number of cobalt in the complex ion [Co(en)Cl4]-? (en = ethylenediamine)
(Multiple Choice)
4.9/5  (36)
(36)
According to valence bond theory,what would be the set of hybrid orbitals used when a Period 4 transition metal forms a tetrahedral complex?
(Multiple Choice)
4.8/5  (39)
(39)
The oxidation and coordination numbers of cobalt in the compound [Co(NH3)5Cl]Cl2 are,respectively:
(Multiple Choice)
4.9/5  (34)
(34)
What geometry is particularly common for complexes of d10 metal ions?
(Short Answer)
4.8/5  (34)
(34)
Showing 1 - 20 of 72
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)