Exam 1: Keys to the Study of Chemistry
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
The result of (3.8621 × 1.5630) - 5.98 is properly written as
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following correctly expresses 52,030.2 m in scientific notation?
(Multiple Choice)
4.9/5  (42)
(42)
The area of a 15-inch pizza is 176.7 in2. Express this area in square centimeters.
(Multiple Choice)
4.9/5  (37)
(37)
The appropriate number of significant figures in the result of 15.234 × 15.208 is
(Multiple Choice)
4.9/5  (29)
(29)
The average distance between the Earth and the Moon is 240,000 miles. Express this distance in kilometers.
(Multiple Choice)
4.7/5  (28)
(28)
The density of mercury, the only metal to exist as a liquid at room temperature, is 13.6 g/cm3. What is that density in pounds per cubic inch?
(Multiple Choice)
4.8/5  (43)
(43)
An evacuated 276 mL glass bulb weighs 129.6375 g. Filled with an unknown gas, the bulb weighs 130.0318 g. Calculate the gas density in g/L, and express it with an appropriate number of significant figures.
(Short Answer)
4.8/5  (35)
(35)
Which of the following abbreviations of the given SI base unit is incorrect?
(Multiple Choice)
4.9/5  (31)
(31)
The speed needed to escape the pull of Earth's gravity is 11.3 km/s. What is this speed in mi/h?
(Multiple Choice)
4.9/5  (33)
(33)
The mass of a sample is 550 milligrams. Which of the following expresses that mass in kilograms?
(Multiple Choice)
4.9/5  (40)
(40)
As part of an experiment to determine the density of a new plastic developed in her laboratory, Sara Ann Dippity measures the volume of a solid sample. Her four trials yield volumes of 12.37 cm3, 12.41 cm3, 12.39 cm3, and 12.38 cm3. Measurements of other scientists in the lab give an average volume of 12.49 cm3. Which of the following statements represents the best analysis of the data?
(Multiple Choice)
4.9/5  (34)
(34)
As chief chemist at Superior Analytical Products (SAP) you must design an experiment to determine the density of an unknown liquid to three (3) significant figures. The density is of the order of 1 g/cm3. You have approximately 7 mL of the liquid and only graduated cylinders and balances are available for your use. Which of the following combinations of equipment will allow you to meet but not exceed your goal?
(Multiple Choice)
4.7/5  (34)
(34)
If the density of a certain spherical atomic nucleus is 1.0 × 1014 g cm-3 and its mass is 2.0 × 10-23 g, what is its radius in cm?
(Multiple Choice)
4.9/5  (21)
(21)
The SI unit of energy is the joule, J. 1 J = 1 kgm2/s2. Another energy unit, the erg, was once in widespread use. 1 erg = 1 gcm2/s2. Calculate the number of ergs in 1 J, showing all your work.
(Short Answer)
4.8/5  (31)
(31)
Which of the following correctly shows how to convert a density of 20.1 g cm-3 to units of kg m-3?
(Multiple Choice)
5.0/5  (25)
(25)
Write the following numbers and results in standard notation, with appropriate significant figures.
a. 7.85 × 10-3
b. 7.85 × 104
c. 5.920 × 103
d. 7.85 × 1012 ÷ 1010
e. 7.00 × 10-5
f. circumference of a circle, 2r, where r = 8.7 cm
g. 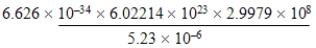
(Essay)
4.9/5  (35)
(35)
Showing 41 - 60 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)