Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Ammonia will react with oxygen in the presence of a copper catalyst to form nitrogen and water. From 164.5°C to 179.0°C, the rate constant increases by a factor of 4.27. What is the activation energy of this oxidation reaction?
(Multiple Choice)
4.8/5  (34)
(34)
Butadiene, C4H6 (used to make synthetic rubber and latex paints) reacts to C8H12 with a rate law of rate = 0.014 L/(mol·s) [C4H6]2. What will be the concentration of C4H6 after 3.0 hours if the initial concentration is 0.025 M?
(Multiple Choice)
4.9/5  (46)
(46)
The rate law for the rearrangement of CH3NC to CH3CN at 800 K is Rate = (1300 s-1)[CH3NC]. What is the half-life for this reaction?
(Multiple Choice)
4.8/5  (38)
(38)
The rate constant for the reaction 3A 4B is 6.00 × 10-3 L mol-1min-1. How long will it take the concentration of A to drop from 0.75 M to 0.25 M?
(Multiple Choice)
4.8/5  (37)
(37)
At 25.0°C, a rate constant has the value 5.21 × 10-8 L mol-1 s-1. If the activation energy is 75.2 kJ/mol, calculate the rate constant when the temperature is 50.0°C.
(Short Answer)
4.9/5  (37)
(37)
Consider the general reaction 5Br-(aq) + BrO3-(aq) + 6H+(aq) 3Br2(aq) + 3H2O(aq)
For this reaction, the rate when expressed as [Br2]/t is the same as
(Multiple Choice)
4.8/5  (45)
(45)
Reaction intermediates differ from activated complexes in that
(Multiple Choice)
4.8/5  (42)
(42)
The half-life of a second-order reaction does not depend on the initial concentration of reactant.
(True/False)
4.9/5  (37)
(37)
Sulfuryl chloride, SO2Cl2(g), decomposes at high temperature to form SO2(g) and Cl2(g). The rate constant at a certain temperature is 4.68 × 10-5s-1. What is the order of the reaction?
(Multiple Choice)
4.8/5  (39)
(39)
The gas-phase reaction CH3NC CH3CN has been studied in a closed vessel, and the rate equation was found to be: Rate = -[CH3NC]/t = k[CH3NC]. Which one of the following actions is least likely to cause a change in the rate of the reaction?
(Multiple Choice)
4.8/5  (35)
(35)
For each of the following terms/concepts, give a brief explanation or definition. Where possible, use examples.
a. order of a reaction
b. elementary reaction
c. reaction intermediate
(Essay)
4.8/5  (33)
(33)
The elementary reaction HBr(g) + Br(g) H(g) + Br2(g) is endothermic.
a. Would you expect the rate constant for the back reaction to be smaller or larger than that for the forward reaction? Explain, briefly.
b. Draw a fully-labeled reaction energy diagram for this reaction, showing the locations of the reactants, products, and transition state.
(Essay)
4.8/5  (35)
(35)
A first-order reaction has a half-life of 20.0 minutes. Starting with 1.00 × 1020 molecules of reactant at time t = 0, how many molecules remain unreacted after 100.0 minutes?
(Multiple Choice)
4.8/5  (40)
(40)
Tetrafluoroethylene, C2F4, can be converted to octafluorocyclobutane which can be used as a refrigerant or an aerosol propellant. A plot of 1/[C2F4] vs. time gives a straight line with a slope of 0.0448 L mol-1s-1. What is the rate law for this reaction?
(Multiple Choice)
4.8/5  (29)
(29)
A reaction has the following rate law: Rate = k[A][B]2
In experiment 1, the concentrations of A and B are both 0.10 mol L-1; in experiment 2, the concentrations are both 0.30 mol L-1. If the temperature stays constant, what is the value of the ratio, Rate(2)/Rate(1)?
(Multiple Choice)
4.8/5  (36)
(36)
In the gas phase at 500.°C, cyclopropane reacts to form propene in a first-order reaction. The figure below shows the concentration of cyclopropane plotted versus time. Use the graph to calculate approximate values of
a. the rate of the reaction, 600. seconds after the start.
b. the half-life of the reaction, t1/2. 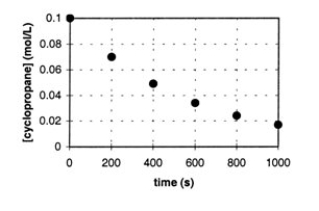
(Essay)
4.9/5  (41)
(41)
An increase in temperature increases the reaction rate because
(Multiple Choice)
5.0/5  (37)
(37)
Chlorine atoms act as heterogeneous catalysts in the destruction of ozone in the stratosphere.
(True/False)
4.9/5  (35)
(35)
Dinitrogen tetraoxide, N2O4, decomposes to nitrogen dioxide, NO2, in a first-order process. If k = 2.5 × 103 s-1 at -5°C and k = 3.5 × 104 s-1 at 25°C, what is the activation energy for the decomposition?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 21 - 40 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)