Exam 19: Oxidation-Reduction Redoxreactions
Exam 2: Matter and Energy50 Questions
Exam 3: Measurement and Chemical Calculations49 Questions
Exam 4: Introduction to Gases49 Questions
Exam 5: Atomic Theory: the Nuclear Model of the Atom46 Questions
Exam 6: Chemical Nomenclature43 Questions
Exam 7: Chemical Formula Relationships44 Questions
Exam 8: Chemical Reactions43 Questions
Exam 9: Chemical Change51 Questions
Exam 10: Quantity Relationships in Chemical Reactions40 Questions
Exam 11: Atomic Theory: the Quantum Model of the Atom50 Questions
Exam 12: Chemical Bonding45 Questions
Exam 13: Structure and Shape47 Questions
Exam 14: The Ideal Gas Law and Its Applications45 Questions
Exam 15: Gases, liquids, and Solids45 Questions
Exam 16: Solutions43 Questions
Exam 17: Acid-Base Proton-Transferreactions45 Questions
Exam 18: Chemical Equilibrium44 Questions
Exam 19: Oxidation-Reduction Redoxreactions44 Questions
Exam 20: Nuclear Chemistry50 Questions
Exam 21: Organic Chemistry45 Questions
Exam 22: Biochemistry45 Questions
Select questions type
Which of the following substances is most likely to be a reducing agent?
(Multiple Choice)
4.9/5  (36)
(36)
In comparing acid-base neutralization reactions and redox reactions,which of the following is correct?
(Multiple Choice)
4.9/5  (37)
(37)
What evidence suggests that Na+ ions are very weak oxidizing agents?
(Multiple Choice)
4.9/5  (38)
(38)
Silver metal will not react with hydrochloric acid.What does this suggest about silver and hydrochloric acid?
(Multiple Choice)
4.8/5  (43)
(43)
Which of the following is the correct net ionic equation for the redox reaction between zinc and the copper(II)ion and the correct direction that is favored? The half-reactions are:
Cu2+ + 2 e- 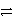 Cu(s)Zn2+ + 2 e-
Cu(s)Zn2+ + 2 e- 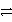 Zn(s)Cu2+ is a stronger oxidizing agent than Zn2+.
Zn(s)Cu2+ is a stronger oxidizing agent than Zn2+.
(Multiple Choice)
4.7/5  (39)
(39)
Which of the following is the correct net ionic equation for the redox reaction between nickel ion and metallic silver and the correct direction that is favored? The half reactions are:
Ag+ + e-  Ag(s)Ni2+ + 2e-
Ag(s)Ni2+ + 2e-  Ni(s)Ag+ is a stronger oxidizing agent than Ni2+.
Ni(s)Ag+ is a stronger oxidizing agent than Ni2+.
(Multiple Choice)
4.9/5  (42)
(42)
Consider the following images.The reactions occurring in each are shown in the choices. 


 A B C D
Which of the following is an example of an electron transfer reaction?
A B C D
Which of the following is an example of an electron transfer reaction?
(Multiple Choice)
4.9/5  (35)
(35)
Match each term with its correct classification:
Proton Donor Proton Acceptor Electron Donor Electron Acceptor
(Multiple Choice)
4.9/5  (46)
(46)
Balance the following redox equation in acidic solution and determine the coefficient placed in front of H2O: ___ Cu + ___ NO3- + ___ H+  ___ Cu2+ + ___ NO + ___ H2O
___ Cu2+ + ___ NO + ___ H2O
(Multiple Choice)
4.9/5  (33)
(33)
In balancing the half-reaction
NO(g)  NO3-(aq)in acidic solution,how many electrons are added,and to which side?
NO3-(aq)in acidic solution,how many electrons are added,and to which side?
(Multiple Choice)
5.0/5  (26)
(26)
Balance the half-reaction in acidic solution and determine the coefficient for hydrogen ion:
___ Cr3+ + ___ H2O  ___ Cr2O72- + ___ H+
___ Cr2O72- + ___ H+
(Multiple Choice)
4.8/5  (40)
(40)
What happens to an elemental oxidizing agent in a redox reaction?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 21 - 40 of 44
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)