Exam 16: A Macroscopic Description of Matter
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions50 Questions
Exam 5: Force and Motion31 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics Ii: Motion in a Plane20 Questions
Exam 9: Impulse and Momentum20 Questions
Exam 10: Energy43 Questions
Exam 11: Work100 Questions
Exam 12: Rotation of a Rigid Body113 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Oscillations49 Questions
Exam 15: Fluids and Elasticity72 Questions
Exam 16: A Macroscopic Description of Matter29 Questions
Exam 17: Work, Heat, and the First Law of Thermodynamics98 Questions
Exam 18: The Micromacro Connection39 Questions
Exam 19: Heat Engines and Refrigerators50 Questions
Exam 20: Traveling Waves49 Questions
Exam 21: Superpositions64 Questions
Exam 22: Wave Optics51 Questions
Exam 23: Ray Optics63 Questions
Exam 24: Optical Instruments49 Questions
Exam 25: Electric Charges and Forces26 Questions
Exam 26: The Electric Field32 Questions
Exam 27: Gausss Law41 Questions
Exam 28: The Electric Potential40 Questions
Exam 29: Potential and Field57 Questions
Exam 30: Current and Resistance32 Questions
Exam 31: Fundamentals of Circuits68 Questions
Exam 32: The Magnetic Field87 Questions
Exam 33: Electromagnetic Induction66 Questions
Exam 34: Electromagnetic Fields and Waves52 Questions
Exam 35: Ac Circuits46 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics8 Questions
Exam 38: Quantization54 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics39 Questions
Exam 42: Nuclear Physics65 Questions
Select questions type
Sometimes an experiment requires a certain pure gas to be used at reduced pressure. One way to achieve this is to purchase a sealed glass container filled with the gas, and to introduce the gas into a vacuum by attaching the glass container to the vacuum chamber and breaking the tip of the glass container using a metallic bean and a magnet. If the volume of the glass container is 1.0 L and it is at a pressure of 1.0 × 105 Pa and if the vacuum chamber has a volume of 2.0 L, what will the pressure be after the gas, which is to be assumed to be an ideal gas, is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
(Multiple Choice)
4.8/5  (24)
(24)
2.0 L of a ideal nitrogen gas (N2) are at 0.00°C and 1.0 atm. The ideal gas constant is R = 8.314 J/mol · K = 0.0821 L ∙ atm/mol ∙ K, Avogadro's number is 6.022 × 1023 molecules/mol, and the ATOMIC mass of nitrogen is 14 g/mol.
(a) Determine the number of moles of N2.
(b) How many molecules of N2 are present?
(c) What is the mass of this gas?
(Essay)
4.8/5  (31)
(31)
The figure shows a pV diagram for 0.95 mol of gas that undergoes the process 1 → 2. The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K. 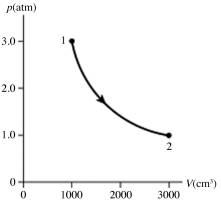
(Multiple Choice)
4.8/5  (38)
(38)
A cold trap is set up to cause molecules to linger near the suction in a vacuum system. If the cold trap has an effective volume of 0.200 L and is maintained at 13.0 K, how many molecules are in it at 10.0 Pa of pressure? (Avogadro's number is 6.022 × 1023 molecules/mol, and the universal gas constant is 8.314 J/mol•K. Assume the behavior of an ideal gas.)
(Multiple Choice)
4.7/5  (28)
(28)
A sealed container holds 0.020 moles of nitrogen (N2) gas, at a pressure of 1.5 atmospheres and a temperature of 290 K. The atomic mass of nitrogen is 14.0 g/mol. The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The mass density of the gas is closest to
(Multiple Choice)
4.8/5  (38)
(38)
A weather balloon contains 12.0 m3 of hydrogen gas when the balloon is released from a location at which the temperature is 22.0°C and the pressure is 101 kPa. The balloon rises to a location where the temperature is -30.0°C and the pressure is 20.0 kPa. If the balloon is free to expand so that the pressure of the gas inside is equal to the ambient pressure, what is the new volume of the balloon? Assume that in both cases the hydrogen gas is in thermal equilibrium with the outside air.
(Multiple Choice)
4.9/5  (45)
(45)
The figure shows a pV diagram for 4.3 g of oxygen gas (O2) in a sealed container. The temperature T1 of the gas in state 1 is 21°C. What are the temperatures T3 and T4 of the gas in states 3 and 4? The ideal gas constant is R = 8.314 J/mol ∙ K = 0.0821 L ∙ atm/mol ∙ K, and the ATOMIC weight of oxygen is 16 g/mol. 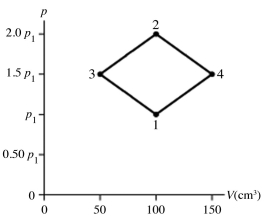
(Multiple Choice)
4.8/5  (35)
(35)
3.00 moles of an ideal gas at a pressure of 250 kPa are held in a container of volume of 25.0 L. The ideal gas constant is R = 8.314 J/mol•K = 0.0821 L ∙ atm/mol ∙ K, and 1 atm = 1.01 x 105 Pa. The temperature of this gas is closest to
(Multiple Choice)
4.9/5  (35)
(35)
Showing 21 - 29 of 29
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)