Exam 3: Stoichiometry of Formulas and Equations
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Lead(II) sulfide was once used in glazing earthenware. It will also react with hydrogen peroxide to form lead(II) sulfate and water. How many grams of hydrogen peroxide are needed to react completely with 265 g of lead(II) sulfide?
(Multiple Choice)
4.8/5  (34)
(34)
Gadolinium oxide, a colorless powder which absorbs carbon dioxide from the air, contains 86.76 mass % Gd. Determine its empirical formula.
(Multiple Choice)
4.9/5  (35)
(35)
In a blast furnace, elemental iron is produced from a mixture of coke (C), iron ore (Fe3O4), and other reactants. An important reaction sequence is 2C(s) + O2(g) → 2CO(g)
Fe3O4(s) + 4CO(g) → 3Fe(l) + 4CO2(g)
How many moles of iron can be formed in this sequence when 1.00 mol of carbon, as coke, is consumed?
(Multiple Choice)
4.8/5  (35)
(35)
Determine the percent composition of potassium dichromate, K2Cr2O7.
(Multiple Choice)
4.8/5  (31)
(31)
In a correctly balanced equation, the number of reactant molecules must equal the number of product molecules.
(True/False)
5.0/5  (32)
(32)
Balance the following equation for the combustion of benzene: C6H6(l) + O2(g) → H2O(g) + CO2(g)
(Multiple Choice)
4.8/5  (36)
(36)
Magnesium fluoride is used in the ceramics and glass industry. What is the mass of 1.72 mol of magnesium fluoride?
(Multiple Choice)
4.8/5  (29)
(29)
Potassium dichromate, K2Cr2O7, is used in tanning leather, decorating porcelain, and water proofing fabrics. Calculate the number of chromium atoms in 78.82 g of K2Cr2O7.
(Multiple Choice)
4.8/5  (25)
(25)
How many grams of oxygen are needed to react completely with 200.0 g of ammonia, NH3? 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
(Multiple Choice)
4.7/5  (25)
(25)
In the combustion analysis of 0.1127 g of glucose (C6H12O6), what mass, in grams, of CO2 would be produced?
(Multiple Choice)
4.8/5  (30)
(30)
An important reaction sequence in the industrial production of nitric acid is the following: N2(g) + 3H2(g) → 2NH3(g)
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(l)
Starting from 20.0 mol of nitrogen gas in the first reaction, how many moles of oxygen gas are required in the second one?
(Multiple Choice)
4.8/5  (21)
(21)
Copper(II) sulfate pentahydrate, CuSO4·5H2O, is used as a fungicide and algicide. Calculate the mass of oxygen in 1.000 mol of CuSO4·5H2O.
(Multiple Choice)
4.8/5  (34)
(34)
Calcium fluoride, CaF2, is a source of fluorine and is used to fluoridate drinking water. Calculate its molar mass.
(Multiple Choice)
4.7/5  (27)
(27)
Lead (II) nitrate is a poisonous substance which has been used in the manufacture of special explosives and as a sensitizer in photography. Calculate the mass of lead in 139 g of Pb(NO3)2.
(Multiple Choice)
5.0/5  (33)
(33)
How many grams of sodium fluoride (used in water fluoridation and manufacture of insecticides) are needed to form 485 g of sulfur tetrafluoride? 3SCl2(l) + 4NaF(s) → SF4(g) + S2Cl2(l) + 4NaCl(s)
(Multiple Choice)
4.7/5  (31)
(31)
Potassium chlorate (used in fireworks, flares, and safety matches) forms oxygen and potassium chloride when heated. KClO3(s) → KCl(s) + O2(g) [unbalanced]
How many grams of oxygen are formed when 26.4 g of potassium chlorate is heated?
(Multiple Choice)
4.8/5  (41)
(41)
Aluminum reacts with oxygen to produce aluminum oxide which can be used as an adsorbent, desiccant, or catalyst for organic reactions. 4Al(s) + 3O2(g) → 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 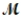 = 26.98 g/mol) and 117.65 g of oxygen (
= 26.98 g/mol) and 117.65 g of oxygen ( 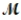 = 32.00 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
= 32.00 g/mol) is allowed to react. Identify the limiting reactant and determine the mass of the excess reactant present in the vessel when the reaction is complete.
(Multiple Choice)
4.9/5  (37)
(37)
Hydroxylamine nitrate contains 29.17 mass % N, 4.20 mass % H, and 66.63 mass % O. Determine its empirical formula.
(Multiple Choice)
4.9/5  (38)
(38)
Showing 41 - 60 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)