Exam 3: Stoichiometry of Formulas and Equations
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Aluminum oxide (used as an adsorbent or a catalyst for organic reactions) forms when aluminum reacts with oxygen. 4Al(s) + 3O2(g) → 2Al2O3(s)
A mixture of 82.49 g of aluminum ( 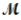 = 26.98 g/mol) and 117.65 g of oxygen (
= 26.98 g/mol) and 117.65 g of oxygen ( 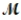 = 32.00g/mol) is allowed to react. What mass of aluminum oxide (
= 32.00g/mol) is allowed to react. What mass of aluminum oxide ( 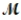 = 101.96 g/mol) can be formed?
= 101.96 g/mol) can be formed?
(Multiple Choice)
4.7/5  (42)
(42)
Sodium bromate is used in a mixture which dissolves gold from its ores. Calculate the mass in grams of 4.68 mol of sodium bromate.
(Multiple Choice)
4.8/5  (23)
(23)
Potassium chloride is used as a substitute for sodium chloride for individuals with high blood pressure. Identify the limiting reactant and determine the mass of the excess reactant remaining when 7.00 g of chlorine gas reacts with 5.00 g of potassium to form potassium chloride.
(Multiple Choice)
4.8/5  (29)
(29)
Balance the following equation: C8H18O3(l) + O2(g) → H2O(g) + CO2(g)
(Multiple Choice)
4.8/5  (38)
(38)
A compound of bromine and fluorine is used to make UF6, which is an important chemical in processing and reprocessing of nuclear fuel. The compound contains 58.37 mass percent bromine. Determine its empirical formula.
(Multiple Choice)
4.9/5  (37)
(37)
Analysis of a carbohydrate showed that it consisted of 40.0 % C, 6.71 % H, and 53.3 % O by mass. Its molecular mass was found to be between 140 and 160 amu. What is the molecular formula of this compound?
(Multiple Choice)
4.9/5  (41)
(41)
Aluminum oxide, Al2O3, is used as a filler for paints and varnishes as well as in the manufacture of electrical insulators. Calculate the number of moles in 47.51 g of Al2O3.
(Multiple Choice)
4.9/5  (33)
(33)
Balance the following equation: UO2(s) + HF(l) → UF4(s) + H2O(l)
(Multiple Choice)
4.7/5  (40)
(40)
Aluminum metal reacts with chlorine gas to form solid aluminum trichloride, AlCl3. What mass of chlorine gas is needed to react completely with 163 g of aluminum?
(Multiple Choice)
4.9/5  (28)
(28)
Ammonia, an important source of fixed nitrogen that can be metabolized by plants, is produced using the Haber process in which nitrogen and hydrogen combine. N2(g) + 3H2(g) → 2NH3(g)
How many grams of nitrogen are needed to produce 325 grams of ammonia?
(Multiple Choice)
4.9/5  (23)
(23)
Balance the following equation: B2O3(s) + HF(l) → BF3(g) + H2O(l)
(Multiple Choice)
4.9/5  (38)
(38)
Tetraphosphorus hexaoxide ( 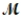 = 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) → P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6, what is the percent yield for the reaction?
= 219.9 g/mol) is formed by the reaction of phosphorus with oxygen gas. P4(s) + 3O2(g) → P4O6(s)
If a mixture of 75.3 g of phosphorus and 38.7 g of oxygen produce 43.3 g of P4O6, what is the percent yield for the reaction?
(Multiple Choice)
4.7/5  (30)
(30)
Household sugar, sucrose, has the molecular formula C12H22O11. What is the percent of carbon in sucrose, by mass?
(Multiple Choice)
4.9/5  (48)
(48)
What is the percent yield for the reaction PCl3(g) + Cl2(g) → PCl5(g)
If 119.3 g of PCl5 (M = 208.2 g/mol) are formed when 61.3 g of Cl2 ( 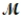 = 70.91 g/mol) react with excess PCl3?
= 70.91 g/mol) react with excess PCl3?
(Multiple Choice)
4.7/5  (23)
(23)
Phosphine, an extremely poisonous and highly reactive gas, will react with oxygen to form tetraphosphorus decaoxide and water. PH3(g) + O2(g) → P4O10(s) + H2O(g) [unbalanced]
Calculate the mass of P4O10(s) formed when 225 g of PH3 reacts with excess oxygen.
(Multiple Choice)
4.7/5  (32)
(32)
Sulfur dioxide reacts with chlorine to produce thionyl chloride (used as a drying agent for inorganic halides) and dichlorine oxide (used as a bleach for wood, pulp, and textiles). SO2(g) + 2Cl2(g) → SOCl2(g) + Cl2O(g)
If 0.400 mol of Cl2 reacts with excess SO2, how many moles of Cl2O are formed?
(Multiple Choice)
4.9/5  (34)
(34)
Phosphorus pentachloride, PCl5, a white solid that has a pungent, unpleasant odor, is used as a catalyst for certain organic reactions. Calculate the number of moles in 38.7 g of PCl5.
(Multiple Choice)
4.9/5  (35)
(35)
A normal breath takes in about 1.0 L of air. Assuming that air has an average molar mass of 28.8g, and that its density is 0.97 g/L, how many molecules of air do you take in with each breath?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following samples has the most moles of the compound?
(Multiple Choice)
4.9/5  (45)
(45)
Showing 21 - 40 of 70
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)