Exam 23: Transition Elements and Their Coordination Compounds
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Which of the following ligands could participate in linkage isomerism?
(Multiple Choice)
4.9/5  (36)
(36)
A feature of transition metal chemistry is that these elements exhibit multiple oxidation states. Which one of the following elements exhibits the smallest number of different oxidation states?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following ligands is most likely to form a high spin octahedral complex with cobalt(II)?
(Multiple Choice)
4.9/5  (34)
(34)
Which of the oxidation states of chromium has the largest valence-state electronegativity?
(Multiple Choice)
4.8/5  (38)
(38)
The oxidation and coordination numbers of cobalt in the compound [Co(NH3)5Cl]Cl2 are,respectively
(Multiple Choice)
4.9/5  (34)
(34)
In the spectrochemical series, which one of the following ligands has the strongest field?
(Multiple Choice)
4.7/5  (33)
(33)
Which of the following coordination numbers applies to octahedral complexes?
(Multiple Choice)
4.9/5  (39)
(39)
Aluminum reacts with oxygen in the air to form a protective oxide coating. Silver also reactswith compounds in air to form a black coating. What substance is formed?
(Multiple Choice)
4.9/5  (25)
(25)
In a coordination compound involving a complex ion of square planar geometry, which of the following types of isomerism is/are never possible?
(Multiple Choice)
4.7/5  (36)
(36)
The maximum oxidation state of an element in the first transition series never exceeds its group number.
(True/False)
4.9/5  (31)
(31)
Which of the following ions is least likely to form colored compounds?
(Multiple Choice)
4.8/5  (31)
(31)
In the presence of a strong octahedral ligand field, the number of unpaired electrons in Co(III) will be
(Multiple Choice)
4.9/5  (30)
(30)
Octahedral complexes can exhibit geometric, optical, and linkage isomerism.
(True/False)
4.9/5  (28)
(28)
Consider the following octahedral complex structures, each involving ethylene diamine and two different, unidentate ligands X and Y. 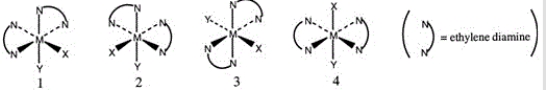 Which one of the following statements about these structures is incorrect?
Which one of the following statements about these structures is incorrect?
(Multiple Choice)
4.8/5  (29)
(29)
In the formation of a transition metal complex, the central metal atom or ion acts as
(Multiple Choice)
4.9/5  (29)
(29)
What is the highest possible oxidation state for molybdenum, Mo?
(Multiple Choice)
4.9/5  (45)
(45)
Showing 41 - 60 of 82
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)