Exam 2: The Components of Matter
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Sodium peroxide is an oxidizer used to bleach animal and vegetable fibers. What is its formula?
(Multiple Choice)
4.9/5  (32)
(32)
Barium fluoride is used in embalming and in glass manufacturing. Which of the following gives the formula and bonding for barium fluoride?
(Multiple Choice)
4.9/5  (38)
(38)
Which one of the following formulas of ionic compounds is the least likely to be correct?
(Multiple Choice)
4.9/5  (35)
(35)
The colorless substance, MgF2, is used in the ceramics and glass industry. What is its name?
(Multiple Choice)
4.8/5  (39)
(39)
Bromine has two naturally-occurring isotopes. 79Br has a mass of 78.9 amu and accounts for 50.3% of bromine atoms. If the atomic mass of bromine is 79.9 amu, what is the mass of an atom of the second bromine isotope?
(Multiple Choice)
4.9/5  (38)
(38)
In the modern periodic table, the order in which the elements are placed is based on
(Multiple Choice)
4.8/5  (39)
(39)
J. J. Thomson studied cathode ray particles (electrons) and was able to measure the mass/charge ratio. His results showed that
(Multiple Choice)
4.7/5  (37)
(37)
For the element bromine, the symbol, group number, group name and physical state are, respectively
(Multiple Choice)
4.8/5  (36)
(36)
What is the chemical symbol for the group 6A (16) element that lies in period 4?
(Multiple Choice)
4.8/5  (33)
(33)
Tetrasulfur dinitride decomposes explosively when heated. What is its formula?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following sets are isoelectronic (i.e., have the same number of electrons)?
i. Br-, Kr, Sr2+
Ii. C, N-, O2-
Iii. Mg2+, Ca2+, Sr2+
Iv. O2-, O, O2+
V. Ag+, Cd2+, Pd
(Multiple Choice)
4.8/5  (40)
(40)
After an atom has lost an electron it becomes a/an ______ and has a _______ charge.
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following symbols does not represent an element?
(Multiple Choice)
4.9/5  (42)
(42)
Bromine is the only nonmetal that is a liquid at room temperature. Consider the isotope bromine-81, 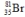 . Select the combination which lists the correct atomic number, neutron number, and mass number, respectively.
. Select the combination which lists the correct atomic number, neutron number, and mass number, respectively.
(Multiple Choice)
4.8/5  (25)
(25)
Showing 81 - 100 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)