Exam 2: The Components of Matter
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Atoms of one element cannot be converted to another element by any known method.
(True/False)
4.8/5  (32)
(32)
Iron (III) chloride hexahydrate is used as a coagulant for sewage and industrial wastes. What is its formula?
(Multiple Choice)
4.7/5  (30)
(30)
The compound, NaH2PO4, is present in many baking powders. What is its name?
(Multiple Choice)
4.8/5  (38)
(38)
Atoms X, Y, Z, and R have the following nuclear compositions: Which two are isotopes? 
(Multiple Choice)
4.8/5  (22)
(22)
The mass of a neutron is equal to the mass of a proton plus the mass of an electron.
(True/False)
4.8/5  (32)
(32)
Who is credited with measuring the mass/charge ratio of the electron?
(Multiple Choice)
4.7/5  (41)
(41)
Silicon, which makes up about 25% of Earth's crust by mass, is used widely in the modern electronics industry. It has three naturally occurring isotopes, 28Si, 29Si, and 30Si. Calculate the atomic mass of silicon. 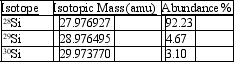
(Multiple Choice)
5.0/5  (35)
(35)
Zinc acetate is used in preserving wood and in manufacturing glazes for porcelain. What is its formula?
(Multiple Choice)
4.8/5  (40)
(40)
What is the name of the acid formed when HClO4 liquid is dissolved in water?
(Multiple Choice)
5.0/5  (33)
(33)
Silver chloride is used in photographic emulsions. What is its formula?
(Multiple Choice)
4.8/5  (35)
(35)
Which one of the following combinations of names and formulas is incorrect?
(Multiple Choice)
4.8/5  (25)
(25)
Chlorine dioxide is a strong oxidizer that is used for bleaching flour and textiles and for purification of water. What is its formula?
(Multiple Choice)
4.8/5  (28)
(28)
Ammonium sulfate, (NH4)2SO4, is a fertilizer widely used as a source of nitrogen. Calculate its molecular mass.
(Multiple Choice)
4.8/5  (31)
(31)
The compound, BaO, absorbs water and carbon dioxide readily and is used to dry gases and organic solvents. What is its name?
(Multiple Choice)
4.8/5  (31)
(31)
Diiodine pentaoxide is used as an oxidizing agent that converts carbon monoxide to carbon dioxide. What is its chemical formula?
(Multiple Choice)
4.8/5  (37)
(37)
Showing 41 - 60 of 100
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)