Exam 24: Carbon: Not Just Another Element
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Cis-1,2-dichloroethylene and trans-1,2-dichloroethylene are examples of _____ isomers.
Free
(Short Answer)
4.9/5  (44)
(44)
Correct Answer:
geometric
Formulas for derivatives of hydrocarbons may be written as R−X,where R is a hydrocarbon lacking a hydrogen atom and X is a functional group.Which of the following formulas represents an ether?
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
D
What is the product of the reduction of butanone?
Free
(Multiple Choice)
4.9/5  (29)
(29)
Correct Answer:
B
The process by which long chain hydrocarbons in petroleum are shortened is called ________.
(Short Answer)
4.7/5  (27)
(27)
Putrescine and cadaverine are two compounds associated with decaying flesh or bad breath.These compounds belong to a class of molecules called ____.
(Multiple Choice)
4.9/5  (37)
(37)
What are the products of a condensation reaction between a primary amine and a carboxylic acid?
(Multiple Choice)
4.7/5  (36)
(36)
Which of the following structural formulas contains an error?
(Multiple Choice)
4.9/5  (30)
(30)
What is the major product upon addition of HBr to 1-hexene?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following reactions is a substitution reaction?
(Multiple Choice)
4.8/5  (30)
(30)
Amino acids polymerize in condensation reactions that result in the formation of an amide linkage (or peptide bond)between amino acid molecules.What dipeptide(s)may be formed in the reaction of glycine with phenylalanine? 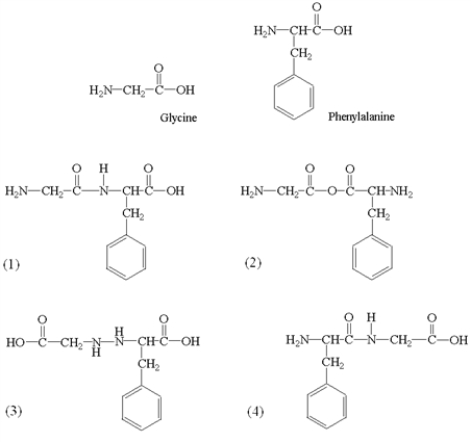
(Multiple Choice)
4.9/5  (33)
(33)
A peptide bond is the amide linkage that is formed in a condensation reaction involving the __________ group of one amino acid with the carboxylic acid group of a second amino acid.
(Short Answer)
4.7/5  (31)
(31)
Which of the following compounds might be used to oxidize an aldehyde to a carboxylic acid?
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following compounds might be used to reduce a carboxylic acid to an aldehyde?
(Multiple Choice)
4.9/5  (42)
(42)
Showing 1 - 20 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)