Exam 16: Principles of Chemical Reactivity: Equilibria
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
What is the Kc equilibrium-constant expression for the following equilibrium? NiO(s)+ H2(g) 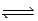 Ni(s)+ H2O(g)
Ni(s)+ H2O(g)
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
A
Which of the following expressions for K is correct for the reaction given below? Al3+(aq)+ 4 OH-(aq) 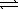 Al(OH)4-(aq)
Al(OH)4-(aq)
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
E
Given the equilibrium constants for the following reactions: 4Cu(s)+ O2(g)  2Cu2O(s),K1
4CuO(s)
2Cu2O(s),K1
4CuO(s)  2Cu2O(s)+ O2(g),K2
What is K for the system
2Cu(s)+ O2(g)
2Cu2O(s)+ O2(g),K2
What is K for the system
2Cu(s)+ O2(g)  2CuO(s)
Equivalent to?
2CuO(s)
Equivalent to?
Free
(Multiple Choice)
4.8/5  (44)
(44)
Correct Answer:
A
What is the expression for Kc for the following equilibrium? CaSO3(s) 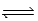 CaO(s)+ SO2(g)
CaO(s)+ SO2(g)
(Multiple Choice)
4.8/5  (41)
(41)
A 2.5 L flask is filled with 0.25 mol SO3,0.20 mol SO2,and 0.40 mol O2,and allowed to reach equilibrium.Assume the temperature of the mixture is chosen so that Kc = 0.12.Predict the effect on the concentration of SO3 as equilibrium is achieved by using Q,the reaction quotient. 2 SO3(g) 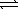 2 SO2(g)+ O2(g)
2 SO2(g)+ O2(g)
(Multiple Choice)
4.7/5  (41)
(41)
For the reaction given below,2.00 moles of A and 3.00 moles of B are placed in a 6.00-L container. A(g)+ 2B(g)  C(g)
At equilibrium,the concentration of A is 0.230 mol/L.What is the value of Kc?
C(g)
At equilibrium,the concentration of A is 0.230 mol/L.What is the value of Kc?
(Multiple Choice)
4.9/5  (42)
(42)
The reaction quotient,Q,for a system is 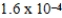 .If the equilibrium constant for the system at some temperature is
.If the equilibrium constant for the system at some temperature is 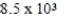 ,what will happen as the reaction mixture returns to equilibrium?
,what will happen as the reaction mixture returns to equilibrium?
(Multiple Choice)
4.9/5  (37)
(37)
When 1.0 mole of acetic acid is diluted with water to a volume of 1.0 L at 25 °C,0.42% of the acetic acid ionizes to form acetate ion and hydronium ion.
CH3CO2H(aq)+ H2O( 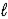 )
) 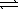 CH3CO2-(aq)+ H3O+(aq)
What percentage of the acid ionizes when 0.75 mole of acetic acid is diluted with water to 1.0 L at 25 °C?
CH3CO2-(aq)+ H3O+(aq)
What percentage of the acid ionizes when 0.75 mole of acetic acid is diluted with water to 1.0 L at 25 °C?
(Short Answer)
4.8/5  (38)
(38)
Which of the following is the correct balanced equation for the equilibrium expression given below? 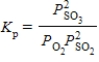
(Multiple Choice)
4.8/5  (44)
(44)
If the reaction quotient,Q,is equal to K in a gas phase reaction,then
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is always true for a reaction where Kc is 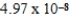 at 25°C?
at 25°C?
(Multiple Choice)
4.8/5  (47)
(47)
In which of the following reactions does a decrease in the volume of the reaction vessel at constant temperature favor the formation of the products?
(Multiple Choice)
4.8/5  (30)
(30)
What is the reaction quotient,Q,for the equilibrium CuCl(s) 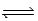 Cu+(aq)+ Cl−(aq)
When 0.3746 L of
Cu+(aq)+ Cl−(aq)
When 0.3746 L of 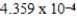 M Cu+ is combined with 0.4326 L of
M Cu+ is combined with 0.4326 L of 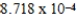 M Cl− in the presence of an excess of CuCl(s)?
M Cl− in the presence of an excess of CuCl(s)?
(Multiple Choice)
4.8/5  (37)
(37)
Consider the reaction A(aq) 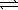 2 B(aq)where Kc = 4.1 at 25 °C.If 0.50 M A(aq)and 1.5 M B(aq)are initially present in a 1.0 L flask at 25 °C,what change in concentrations (if any)will occur in time?
2 B(aq)where Kc = 4.1 at 25 °C.If 0.50 M A(aq)and 1.5 M B(aq)are initially present in a 1.0 L flask at 25 °C,what change in concentrations (if any)will occur in time?
(Multiple Choice)
4.8/5  (35)
(35)
The equilibrium constant (Kc)for the decomposition of ammonium hydrogen sulfide,NH4HS(s) 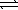 NH3(g)+ H2S(g),is 1.8 × 10-4 at 25 °C.If excess NH4HS(s)is allowed to equilibrate at 25 °C,what is the equilibrium concentration of NH3?
NH3(g)+ H2S(g),is 1.8 × 10-4 at 25 °C.If excess NH4HS(s)is allowed to equilibrate at 25 °C,what is the equilibrium concentration of NH3?
(Multiple Choice)
4.8/5  (33)
(33)
In 1913,the Haber-Bosch process was patented.The product of the Haber-Bosch process is ________.
(Short Answer)
4.9/5  (37)
(37)
For the equilibrium PCl5(g) 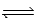 PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.19 M,what is the equilibrium concentration of PCl3?
PCl3(g)+ Cl2(g),Kc = 4.0 at 228°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl5(g)is 0.19 M,what is the equilibrium concentration of PCl3?
(Multiple Choice)
4.8/5  (29)
(29)
At a given temperature,K = 0.024 for the equilibrium: PCl5(g)  PCl3(g)+ Cl2(g)
What is K for:
Cl2(g)+ PCl3(g)
PCl3(g)+ Cl2(g)
What is K for:
Cl2(g)+ PCl3(g)  PCl5(g)?
PCl5(g)?
(Multiple Choice)
4.9/5  (41)
(41)
Write a balanced chemical equation which corresponds to the following equilibrium constant expression. 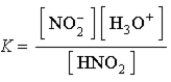
(Multiple Choice)
4.9/5  (33)
(33)
At a given temperature,0.0664 mol N2O4(g)is placed in a 1.00 L flask.After reaching equilibrium,the concentration of NO2(g)is 6.1 × 10-3 M.What is Kc for the reaction below? N2O4(g) 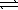 2 NO2(g)
2 NO2(g)
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)